Azithromycin Causes a Novel Proarrhythmic Syndrome
- PMID: 28408648
- PMCID: PMC5396181
- DOI: 10.1161/CIRCEP.115.003560
Azithromycin Causes a Novel Proarrhythmic Syndrome
Abstract
Background: The widely used macrolide antibiotic azithromycin increases risk of cardiovascular and sudden cardiac death, although the underlying mechanisms are unclear. Case reports, including the one we document here, demonstrate that azithromycin can cause rapid, polymorphic ventricular tachycardia in the absence of QT prolongation, indicating a novel proarrhythmic syndrome. We investigated the electrophysiological effects of azithromycin in vivo and in vitro using mice, cardiomyocytes, and human ion channels heterologously expressed in human embryonic kidney (HEK 293) and Chinese hamster ovary (CHO) cells.
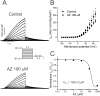
Methods and results: In conscious telemetered mice, acute intraperitoneal and oral administration of azithromycin caused effects consistent with multi-ion channel block, with significant sinus slowing and increased PR, QRS, QT, and QTc intervals, as seen with azithromycin overdose. Similarly, in HL-1 cardiomyocytes, the drug slowed sinus automaticity, reduced phase 0 upstroke slope, and prolonged action potential duration. Acute exposure to azithromycin reduced peak SCN5A currents in HEK cells (IC50=110±3 μmol/L) and Na+ current in mouse ventricular myocytes. However, with chronic (24 hour) exposure, azithromycin caused a ≈2-fold increase in both peak and late SCN5A currents, with findings confirmed for INa in cardiomyocytes. Mild block occurred for K+ currents representing IKr (CHO cells expressing hERG; IC50=219±21 μmol/L) and IKs (CHO cells expressing KCNQ1+KCNE1; IC50=184±12 μmol/L), whereas azithromycin suppressed L-type Ca++ currents (rabbit ventricular myocytes, IC50=66.5±4 μmol/L) and IK1 (HEK cells expressing Kir2.1, IC50=44±3 μmol/L).
Conclusions: Chronic exposure to azithromycin increases cardiac Na+ current to promote intracellular Na+ loading, providing a potential mechanistic basis for the novel form of proarrhythmia seen with this macrolide antibiotic.
Keywords: calcium channel; mice; pharmacology; potassium channels; sodium channels.
© 2017 American Heart Association, Inc.
Figures







Comment in
-
Drug-Induced Arrhythmias, Precision Medicine, and Small Data.Circ Arrhythm Electrophysiol. 2017 Apr;10(4):e005208. doi: 10.1161/CIRCEP.117.005208. Circ Arrhythm Electrophysiol. 2017. PMID: 28408653 Free PMC article. No abstract available.
References
-
- Roden DM. Clinical practice. Long-QT syndrome. N Engl J Med. 2008;358:169–176. - PubMed
-
- Owens RC, Jr, Nolin TD. Antimicrobial-associated QT interval prolongation: Pointes of interest. Clin Infect Dis. 2006;43:1603–1611. - PubMed
-
- Arellano-Rodrigo E, Garcia A, Mont L, Roque M. Torsade de pointes and cardiorespiratory arrest induced by azithromycin in a patient with congenital long QT syndrome. Med Clin (Barc) 2001;117:118–119. - PubMed
-
- Huang BH, Wu CH, Hsia CP, Yin CC. Azithromycin-induced torsade de pointes. Pacing Clin Electrophysiol. 2007;30:1579–1582. - PubMed
-
- Kezerashvili A, Khattak H, Barsky A, Nazari R, Fisher JD. Azithromycin as a cause of QT-interval prolongation and torsade de pointes in the absence of other known precipitating factors. J Interv Card Electrophysiol. 2007;18:243–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL049989/HL/NHLBI NIH HHS/United States
- R01 HL124935/HL/NHLBI NIH HHS/United States
- T32 GM007569/GM/NIGMS NIH HHS/United States
- U19 HL065962/HL/NHLBI NIH HHS/United States
- U01 HL065962/HL/NHLBI NIH HHS/United States
- R01 HL088635/HL/NHLBI NIH HHS/United States
- R01 HL096844/HL/NHLBI NIH HHS/United States
- R01 HL118952/HL/NHLBI NIH HHS/United States
- R01 HL133127/HL/NHLBI NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- R21 HL108037/HL/NHLBI NIH HHS/United States
- F30 HL127962/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

