Premature Ventricular Contraction Coupling Interval Variability Destabilizes Cardiac Neuronal and Electrophysiological Control: Insights From Simultaneous Cardioneural Mapping
- PMID: 28408652
- PMCID: PMC5407491
- DOI: 10.1161/CIRCEP.116.004937
Premature Ventricular Contraction Coupling Interval Variability Destabilizes Cardiac Neuronal and Electrophysiological Control: Insights From Simultaneous Cardioneural Mapping
Abstract
Background: Variability in premature ventricular contraction (PVC) coupling interval (CI) increases the risk of cardiomyopathy and sudden death. The autonomic nervous system regulates cardiac electrical and mechanical indices, and its dysregulation plays an important role in cardiac disease pathogenesis. The impact of PVCs on the intrinsic cardiac nervous system, a neural network on the heart, remains unknown. The objective was to determine the effect of PVCs and CI on intrinsic cardiac nervous system function in generating cardiac neuronal and electric instability using a novel cardioneural mapping approach.
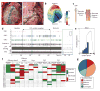
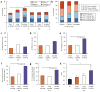
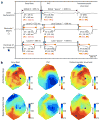
Methods and results: In a porcine model (n=8), neuronal activity was recorded from a ventricular ganglion using a microelectrode array, and cardiac electrophysiological mapping was performed. Neurons were functionally classified based on their response to afferent and efferent cardiovascular stimuli, with neurons that responded to both defined as convergent (local reflex processors). Dynamic changes in neuronal activity were then evaluated in response to right ventricular outflow tract PVCs with fixed short, fixed long, and variable CI. PVC delivery elicited a greater neuronal response than all other stimuli (P<0.001). Compared with fixed short and long CI, PVCs with variable CI had a greater impact on neuronal response (P<0.05 versus short CI), particularly on convergent neurons (P<0.05), as well as neurons receiving sympathetic (P<0.05) and parasympathetic input (P<0.05). The greatest cardiac electric instability was also observed after variable (short) CI PVCs.
Conclusions: Variable CI PVCs affect critical populations of intrinsic cardiac nervous system neurons and alter cardiac repolarization. These changes may be critical for arrhythmogenesis and remodeling, leading to cardiomyopathy.
Keywords: PVC-induced cardiomyopathy; autonomic nervous system; coupling; intrinsic cardiac ganglia; neurocardiology; premature ventricular beats.
© 2017 American Heart Association, Inc.
Figures
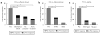


References
-
- Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, Tan V, Lerman BB, Mittal S. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. - PubMed
-
- Leenhardt A, Glaser E, Burguera M, Nurnberg M, Maison-Blanche P, Coumel P. Short-coupled variant of torsade de pointes. A new electrocardiographic entity in the spectrum of idiopathic ventricular tachyarrhythmias. Circulation. 1994;89:206–215. - PubMed
-
- Viskin S, Rosso R, Rogowski O, Belhassen B. The “short-coupled” variant of right ventricular outflow ventricular tachycardia: a not-so-benign form of benign ventricular tachycardia? J Cardiovasc Electrophysiol. 2005;16:912–916. - PubMed
-
- Kawamura M, Badhwar N, Vedantham V, Tseng ZH, Lee BK, Lee RJ, Marcus GM, Olgin JE, Gerstenfeld EP, Scheinman MM. Coupling interval dispersion and body mass index are independent predictors of idiopathic premature ventricular complex-induced cardiomyopathy. J Cardiovasc Electrophysiol. 2014;25:756–762. - PubMed
-
- Bradfield JS, Homsi M, Shivkumar K, Miller JM. Coupling interval variability differentiates ventricular ectopic complexes arising in the aortic sinus of valsalva and great cardiac vein from other sources: mechanistic and arrhythmic risk implications. J Am Coll Cardiol. 2014;63:2151–2158. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

