Multi-Omics of Tomato Glandular Trichomes Reveals Distinct Features of Central Carbon Metabolism Supporting High Productivity of Specialized Metabolites
- PMID: 28408661
- PMCID: PMC5466034
- DOI: 10.1105/tpc.17.00060
Multi-Omics of Tomato Glandular Trichomes Reveals Distinct Features of Central Carbon Metabolism Supporting High Productivity of Specialized Metabolites
Abstract
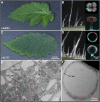
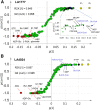
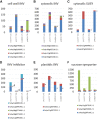
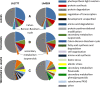
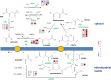
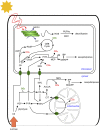
Glandular trichomes are metabolic cell factories with the capacity to produce large quantities of secondary metabolites. Little is known about the connection between central carbon metabolism and metabolic productivity for secondary metabolites in glandular trichomes. To address this gap in our knowledge, we performed comparative metabolomics, transcriptomics, proteomics, and 13C-labeling of type VI glandular trichomes and leaves from a cultivated (Solanum lycopersicum LA4024) and a wild (Solanum habrochaites LA1777) tomato accession. Specific features of glandular trichomes that drive the formation of secondary metabolites could be identified. Tomato type VI trichomes are photosynthetic but acquire their carbon essentially from leaf sucrose. The energy and reducing power from photosynthesis are used to support the biosynthesis of secondary metabolites, while the comparatively reduced Calvin-Benson-Bassham cycle activity may be involved in recycling metabolic CO2 Glandular trichomes cope with oxidative stress by producing high levels of polyunsaturated fatty acids, oxylipins, and glutathione. Finally, distinct mechanisms are present in glandular trichomes to increase the supply of precursors for the isoprenoid pathways. Particularly, the citrate-malate shuttle supplies cytosolic acetyl-CoA and plastidic glycolysis and malic enzyme support the formation of plastidic pyruvate. A model is proposed on how glandular trichomes achieve high metabolic productivity.
© 2017 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Balcke G.U., Bennewitz S., Zabel S., Tissier A. (2014). Isoprenoid and metabolite profiling of plant trichomes. Methods Mol. Biol. 1153: 189–202. - PubMed
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Met. 57: 289–300.
-
- Bleeker P.M., Mirabella R., Diergaarde P.J., VanDoorn A., Tissier A., Kant M.R., Prins M., de Vos M., Haring M.A., Schuurink R.C. (2012). Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 109: 20124–20129. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

