Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury
- PMID: 28408732
- PMCID: PMC5400030
- DOI: 10.12659/msm.902754
Glial Cell Line-Derived Neurotrophic Factor-Transfected Placenta-Derived Versus Bone Marrow-Derived Mesenchymal Cells for Treating Spinal Cord Injury
Abstract
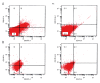
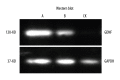
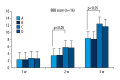
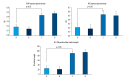
BACKGROUND Placenta-derived mesenchymal stem cells (PMSCs) were isolated from placenta and had differentiation and self-renewal potential. We transfected PMSCs with glial cell line-derived neurotrophic factor (GDNF) and compared their effect for repairing spinal cord injury (SCI) with that of GDNF-transfected bone marrow-derived mesenchymal stem cell (BMSC). MATERIAL AND METHODS The PMSCs were isolated from Sprague-Dawley rat placenta; BMSCs were isolated from Sprague-Dawley rat thigh bone marrow. Primary cultured BMSCs and PMSCs were uniformly spindle-shaped. Flow cytometry indicated that both cell types were CD29- and CD90-positive and CD34- and CD45-negative, confirming that they were MSCs. The PMSCs and BMSCs were transfected with recombinant lentivirus containing the GDNF gene in vitro. PMSC and BMSC viability was increased after transfection, and GDNF expression was increased until 10 d after transfection. SCI was created in the rats (n=64) and was repaired using transfected PMSCs and BMSCs or untransfected PMSCs and BMSCs. RESULTS The transfected PMSCs and BMSCs repaired the SCI. Flow cytometry, histology, immunohistochemical, kinesiology properties, and Basso-Beattie-Bresnahan locomotion score measurements determined no significant difference between transfected PMSCs and BMSCs at 7, 14, and 21 d post-transplantation (P>0.05); the injury healed better in transfected PMSCs and BMSCs than in untransfected PMSCs and BMSCs (P<0.05). CONCLUSIONS MSCs have similar biology characteristics and capacity for SCI repair to BMSCs and can be used as a new resource for treating SCI.
Figures




References
-
- Anderberg L, Aldskogius H, Holtz A. Spinal cord injury – scientific challenges for the unknown future. Ups J Med Sci. 2007;112:259–88. - PubMed
-
- Bae JS, Han HS, Youn DH. Bone marrow-derived mesenchymal stem cells promote neuronal networks with functional synaptic transmission after transplantation into mice with neurodegeneration. Stem Cells. 2007;5:1307–16. - PubMed
-
- Lu D, Li Y, Wang L. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;8:813–19. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

