Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway
- PMID: 28408737
- PMCID: PMC5429837
- DOI: 10.1038/s41598-017-01046-2
Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway
Abstract
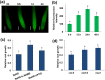
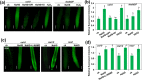
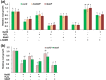
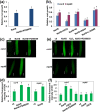
High concentrations of hydrogen sulfide (H2S) are toxic to plants and inhibit their growth. Previous research indicated that high concentrations of H2S modulate the root system architecture (RSA) by affecting auxin transport; however, the signaling pathway underlying this process remains unclear. Here, we investigated the effects of exogenous sodium hydrosulfide (NaHS), an H2S donor, on primary root (PR) growth in Arabidopsis using pharmacological, physiological, and genetic approaches. H2S toxicity repressed PR growth by triggering a signal transduction pathway involving reactive oxygen species (ROS) accumulation, MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6) activation, and nitric oxide (NO) production. Respiratory burst oxidase homolog mutants and an NO synthase mutant were less sensitive to NaHS, suggesting that both ROS and NO mediate the inhibitory effects of H2S on PR growth. We found that exogenous H2S-activated ROS production was required for NO generation and that MPK6 mediated H2S-induced NO production. MPK6 was shown to function downstream of ROS and upstream of NO. Finally, we demonstrated that exogenous H2S repressed the distribution of auxin and reduced the meristematic cell division potential in root tips, and NO was involved in this process.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013;64:1953–1966. doi: 10.1093/jxb/ert055. - DOI - PMC - PubMed
-
- Chen J, et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil. 2013;362:301–318. doi: 10.1007/s11104-012-1275-7. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

