APAs Constraints to Voluntary Movements: The Case for Limb Movements Coupling
- PMID: 28408875
- PMCID: PMC5374888
- DOI: 10.3389/fnhum.2017.00152
APAs Constraints to Voluntary Movements: The Case for Limb Movements Coupling
Abstract
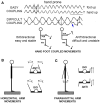
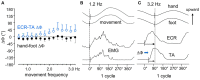
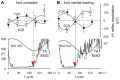
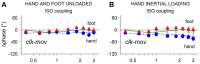
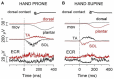
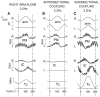
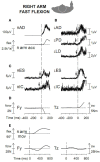
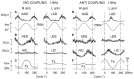
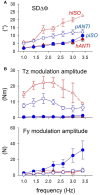
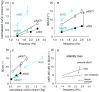
When rhythmically moving two limbs in either the same or in opposite directions, one coupling mode meets constraints that are absent in the other mode. Isodirectional (ISO) flexion-extensions of the ipsilateral hand and foot can be easily performed with either the hand prone or supine. Instead, antidirectional (ANTI) movements require attentive effort and irresistibly tend to reverse into ISO when frequency increases. Experimental evidence indicates that the direction dependent easy-difficult dichotomy is caused by interference of the anticipatory postural commands associated to movements of one limb with voluntary commands to the other limb. Excitability of the resting wrist muscles is subliminally modulated at the period of ipsilateral foot oscillations, being phase-opposite in the antagonists and distributed so as to facilitate ISO and obstacle ANTI coupling of the hand (either prone or supine) with the foot. Modulation is driven by cortical signals dispatched to the forearm simultaneously with the voluntary commands moving the foot. If right foot oscillations are performed when standing on the left foot with the right hand touching a fixed support, the subliminal excitability modulation is replaced by overt contractions of forearm muscles conforming the APAs features. This suggests that during hand-foot ANTI coupling the voluntary commands to forearm muscles are contrasted by APAs commands of opposite sign linked to foot oscillations. Correlation between the easy-difficult dichotomy and the APAs distribution is also found in coupled adduction-abduction of the arms or hands in the transverse plane and in coupled flexion-extension of the arms in the parasagittal plane. In all these movements, APAs commands linked to the movement of each limb reach the motor pathways to the contralateral muscles homologous to the prime movers and can interfere during coupling with their voluntary activation. APAs are also generated in postural muscles of trunk and lower limbs and size-increase when the movement frequency is incremented. The related increase in postural effort apparently contributes in destabilizing the difficult coupling mode. Motor learning may rely upon more effective APAs. APAs and focal contraction are entangled within the same voluntary action. Yet, neural diseases may selectively impair APAs, which represent a potential target for rehabilitation.
Keywords: APAs; APAs destabilizing effects on coupling; coupled movements synchronization; direction principle; in phase and antiphase coupling; limb movements coupling; motor learning/training.
Figures


References
-
- Babinski J. (1899). De l' asynergie cérébelleuse. Rev. Neurol. 7, 806–816.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

