Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response
- PMID: 28408906
- PMCID: PMC5374155
- DOI: 10.3389/fimmu.2017.00254
Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response
Abstract
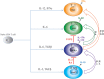
After emerging from the thymus, naive CD4 T cells circulate through secondary lymphoid tissues, including gut-associated lymphoid tissue of the intestine. The activation of naïve CD4 T cells by antigen-presenting cells offering cognate antigen initiate differentiation programs that lead to the development of highly specialized T helper (Th) cell lineages. Although initially believed that developmental programing of effector T cells such as T helper 1 (Th1) or T helper 2 (Th2) resulted in irreversible commitment to a fixed fate, subsequent studies have demonstrated greater flexibility, or plasticity, in effector T cell stability than originally conceived. This is particularly so for the Th17 subset, differentiation of which is a highly dynamic process with overlapping developmental axes with inducible regulatory T (iTreg), T helper 22 (Th22), and Th1 cells. Accordingly, intermediary stages of Th17 cells are found in various tissues, which co-express lineage-specific transcription factor(s) or cytokine(s) of developmentally related CD4 T cell subsets. A highly specialized tissue like that of the intestine, which harbors the largest immune compartment of the body, adds several layers of complexity to the intricate process of Th differentiation. Due to constant exposure to millions of commensal microbes and periodic exposure to pathogens, the intestinal mucosa maintains a delicate balance between regulatory and effector T cells. It is becoming increasingly clear that equilibrium between tolerogenic and inflammatory axes is maintained in the intestine by shuttling the flexible genetic programming of a developing CD4 T cell along the developmental axis of iTreg, Th17, Th22, and Th1 subsets. Currently, Th17 plasticity remains an unresolved concern in the field of clinical research as targeting Th17 cells to cure immune-mediated disease might also target its related subsets. In this review, we discuss the expanding sphere of Th17 plasticity through its shared developmental axes with related cellular subsets such as Th22, Th1, and iTreg in the context of intestinal inflammation and also examine the molecular and epigenetic features of Th17 cells that mediate these overlapping developmental programs.
Keywords: T helper cells; Th17; developmental plasticity; immune response; intestine.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

