Activities of Amphioxus GH-Like Protein in Osmoregulation: Insight into Origin of Vertebrate GH Family
- PMID: 28408927
- PMCID: PMC5376476
- DOI: 10.1155/2017/9538685
Activities of Amphioxus GH-Like Protein in Osmoregulation: Insight into Origin of Vertebrate GH Family
Abstract
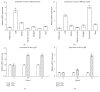
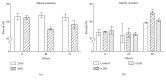
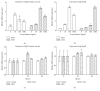
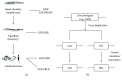
GH is known to play an important role in both growth promotion and osmoregulation in vertebrates. We have shown that amphioxus possesses a single GH-like hormone (GHl) gene encoding a functional protein capable of promoting growth. However, if GHl can mediate osmoregulation remains open. Here, we demonstrated clearly that GHl increased not only the survival rate of amphioxus but also the muscle moisture under high salinity. Moreover, GHl induced the expression of both the ion transporter Na+-K+-ATPase (NKA) and Na+-K+-2Cl- cotransporter (NKCC) in the gill as well as the mediator of GH action IGFl in the hepatic caecum, indicating that GHl fulfills this osmoregulatory activity through the same mechanisms of vertebrate GH. These results together suggest that the osmoregulatory activities of GH had emerged in the basal chordate amphioxus. We also proposed a new model depicting the origin of pituitary hormone family in vertebrates.
Figures




Similar articles
-
The Origination of Growth Hormone/Insulin-Like Growth Factor System: A Story From Ancient Basal Chordate Amphioxus.Front Endocrinol (Lausanne). 2022 Apr 1;13:825722. doi: 10.3389/fendo.2022.825722. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432211 Free PMC article. Review.
-
Functional characterization of GH-like homolog in amphioxus reveals an ancient origin of GH/GH receptor system.Endocrinology. 2014 Dec;155(12):4818-30. doi: 10.1210/en.2014-1377. Epub 2014 Oct 21. Endocrinology. 2014. PMID: 25333966
-
Expression and regulation by thyroid hormone (TH) of zebrafish IGF-I gene and amphioxus IGFl gene with implication of the origin of TH/IGF signaling pathway.Comp Biochem Physiol A Mol Integr Physiol. 2011 Dec;160(4):474-9. doi: 10.1016/j.cbpa.2011.08.005. Epub 2011 Aug 16. Comp Biochem Physiol A Mol Integr Physiol. 2011. PMID: 21867768
-
Osmoregulation in the Plotosidae Catfish: Role of the Salt Secreting Dendritic Organ.Front Physiol. 2018 Jul 3;9:761. doi: 10.3389/fphys.2018.00761. eCollection 2018. Front Physiol. 2018. PMID: 30018560 Free PMC article.
-
[Hepatic caecum of amphioxus and origin of vertebrate liver].Yi Chuan. 2010 May;32(5):437-42. doi: 10.3724/sp.j.1005.2010.00437. Yi Chuan. 2010. PMID: 20466630 Review. Chinese.
Cited by
-
PACAP/GCGa Is an Important Modulator of the Amphioxus CNS-Hatschek's Pit Axis, the Homolog of the Vertebrate Hypothalamic-Pituitary Axis in the Basal Chordates.Front Endocrinol (Lausanne). 2022 Apr 14;13:850040. doi: 10.3389/fendo.2022.850040. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35498398 Free PMC article.
-
Ion regulation at gills precedes gas exchange and the origin of vertebrates.Nature. 2022 Oct;610(7933):699-703. doi: 10.1038/s41586-022-05331-7. Epub 2022 Oct 19. Nature. 2022. PMID: 36261526
-
The Origination of Growth Hormone/Insulin-Like Growth Factor System: A Story From Ancient Basal Chordate Amphioxus.Front Endocrinol (Lausanne). 2022 Apr 1;13:825722. doi: 10.3389/fendo.2022.825722. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35432211 Free PMC article. Review.
-
Optimum salinity for Nile tilapia (Oreochromis niloticus) growth and mRNA transcripts of ion-regulation, inflammatory, stress- and immune-related genes.Fish Physiol Biochem. 2019 Aug;45(4):1217-1232. doi: 10.1007/s10695-019-00640-7. Epub 2019 May 8. Fish Physiol Biochem. 2019. PMID: 31069608
-
Deep whole-genome resequencing sheds light on the distribution and effect of amphioxus SNPs.BMC Genom Data. 2022 Apr 8;23(1):26. doi: 10.1186/s12863-022-01038-w. BMC Genom Data. 2022. PMID: 35395709 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

