Tissue Distribution of trans-Resveratrol and Its Metabolites after Oral Administration in Human Eyes
- PMID: 28409021
- PMCID: PMC5377058
- DOI: 10.1155/2017/4052094
Tissue Distribution of trans-Resveratrol and Its Metabolites after Oral Administration in Human Eyes
Abstract
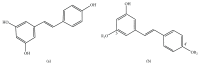
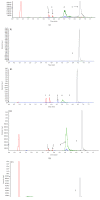
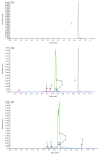
Purpose. This study was performed to measure the concentration of trans-resveratrol and its three metabolites in human eyes. Methods. The patients who underwent pars plana vitrectomy for rhegmatogenous retinal detachment were included. The participants were orally given trans-resveratrol-based supplement (Longevinex®). A suitable amount of conjunctiva, aqueous humor, and vitreous humor were obtained during the operation. High-performance liquid chromatography (HPLC) with mass spectrometry (LC/MS/MS) was used to detect the concentration of trans-resveratrol and its three metabolites in the various samples. Results. The average concentration of resveratrol in the conjunctiva was 17.19 ± 15.32 nmol/g (mean ± SD). The concentration of resveratrol in the aqueous humor was close to the limit of detection, but its metabolites could be quantified. The concentrations of resveratrol metabolites in the aqueous humor can be detected. In the vitreous humor, the average concentration of resveratrol-3-O-sulfate was 62.95 ± 41.97 nmol/L. The sulfate conjugations of resveratrol were recovered in the conjunctiva, aqueous humor, and vitreous humor. Conclusions. Resveratrol and its three metabolites can be detected in the ocular tissues after oral administration. Although the concentration of parent resveratrol was low in the eyes, its metabolites could be detected and may have a role in the treatment of ocular diseases.
Figures
Similar articles
-
Penetration of ofloxacin in human aqueous and vitreous humors following oral and topical administration.Retina. 1998;18(6):521-5. doi: 10.1097/00006982-199806000-00005. Retina. 1998. PMID: 9869460
-
Vitreous and aqueous concentrations of brimonidine following topical application of brimonidine tartrate 0.1% ophthalmic solution in humans.J Ocul Pharmacol Ther. 2015 Jun;31(5):282-5. doi: 10.1089/jop.2015.0003. Epub 2015 Apr 28. J Ocul Pharmacol Ther. 2015. PMID: 25918904 Clinical Trial.
-
Aqueous and vitreous penetration of levofloxacin after topical and/or oral administration.Eur J Ophthalmol. 2007 May-Jun;17(3):372-6. doi: 10.1177/112067210701700316. Eur J Ophthalmol. 2007. PMID: 17534819 Clinical Trial.
-
Vitreous and Aqueous Penetration of Orally Administered Trimethoprim-Sulfamethoxazole Combination in Humans.Cornea. 2013 Oct;32(10):1315-20. doi: 10.1097/ICO.0b013e318298ddf8. Cornea. 2013. PMID: 23928948
-
Quantification of trans-resveratrol and its metabolites in human plasma using ultra-high performance liquid chromatography tandem quadrupole-orbitrap mass spectrometry.J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Jan 1;1104:119-129. doi: 10.1016/j.jchromb.2018.11.016. Epub 2018 Nov 13. J Chromatogr B Analyt Technol Biomed Life Sci. 2019. PMID: 30453129
Cited by
-
Role of Resveratrol in Prevention and Control of Cardiovascular Disorders and Cardiovascular Complications Related to COVID-19 Disease: Mode of Action and Approaches Explored to Increase Its Bioavailability.Molecules. 2021 May 11;26(10):2834. doi: 10.3390/molecules26102834. Molecules. 2021. PMID: 34064568 Free PMC article. Review.
-
RESVEGA, a Nutraceutical Omega-3/Resveratrol Supplementation, Reduces Angiogenesis in a Preclinical Mouse Model of Choroidal Neovascularization.Int J Mol Sci. 2021 Oct 13;22(20):11023. doi: 10.3390/ijms222011023. Int J Mol Sci. 2021. PMID: 34681683 Free PMC article.
-
Anti-Inflammatory Effects of Resveratrol on Human Retinal Pigment Cells and a Myopia Animal Model.Curr Issues Mol Biol. 2021 Jul 16;43(2):716-727. doi: 10.3390/cimb43020052. Curr Issues Mol Biol. 2021. PMID: 34287272 Free PMC article.
-
Significance of Resveratrol in Clinical Management of Chronic Diseases.Molecules. 2017 Aug 18;22(8):1329. doi: 10.3390/molecules22081329. Molecules. 2017. PMID: 28820474 Free PMC article. Review.
-
Natural Compounds as Promising Adjuvant Agents in The Treatment of Gliomas.Int J Mol Sci. 2022 Mar 20;23(6):3360. doi: 10.3390/ijms23063360. Int J Mol Sci. 2022. PMID: 35328780 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

