The SMARTER pilot study: Testing feasibility of real-time feedback for dietary self-monitoring
- PMID: 28409090
- PMCID: PMC5388931
- DOI: 10.1016/j.pmedr.2017.03.017
The SMARTER pilot study: Testing feasibility of real-time feedback for dietary self-monitoring
Abstract
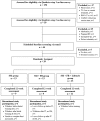
Self-monitoring (SM) of food intake is central to weight loss treatment. Technology makes it possible to reinforce this behavior change strategy by providing real-time feedback (FB) tailored to the diary entry. To test the feasibility of providing 1-4 daily FB messages tailored to dietary recordings via a smartphone, we conducted a 12-week pilot randomized clinical trial in Pittsburgh, PA in US in 2015. We compared 3 groups: SM using the Lose It! smartphone app (Group 1); SM + FB (Group 2); and SM + FB + attending three in-person group sessions (Group 3). The sample (N = 39) was mostly white and female with a mean body mass index of 33.76 kg/m2. Adherence to dietary SM was recorded daily, weight was assessed at baseline and 12 weeks. The mean percentage of days adherent to dietary SM was similar among Groups 1, 2, and 3 (p = 0.66) at 53.50% vs. 55.86% vs. 65.33%, respectively. At 12 weeks, all groups had a significant percent weight loss (p < 0.05), with no differences among groups (- 2.85% vs. - 3.14% vs. - 3.37%) (p = 0.95); 26% of the participants lost ≥ 5% of their baseline weight. Mean retention was 74% with no differences among groups (p = 0.37). All groups adhered to SM at levels comparable to or better than other weight loss studies and lost acceptable amounts of weight, with minimal intervention contact over 12 weeks. These preliminary findings suggest this 3-group approach testing SM alone vs. SM with real-time FB messages alone or supplemented with limited in-person group sessions warrants further testing in a larger, more diverse sample and for a longer intervention period.
Keywords: Feedback; Mobile technology; Obesity; Overweight; Self-efficacy; Self-monitoring; Standard behavioral treatment; Weight loss; mHealth.
Figures
References
-
- Ambeba E.J., Sereika S.M., Styn M.A. The effect of tailored feedback messages, delivered remotely and daily, on changes in dietary intake and measures of adiposity. Journal of Patient Education and Counseling. 2012
-
- Burke L.E., Styn M.A., Steenkiste A.R., Music E., Warziski M., Choo J. A randomized clinical trial testing treatment preference and two dietary options in behavioral weight management: preliminary results of the impact of diet at 6 months—PREFER study. Obesity (Silver Spring) 2006;14(11):2007–2017. - PubMed
-
- Burke L.E., Warziski M., Acharya S. PREFER trial: a randomized clinical trial testing treatment preference and two dietary options combined with behavioral weight management. Obesity. 2006;14(Suppl):A32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources