PGC1 α Activators Mitigate Diabetic Tubulopathy by Improving Mitochondrial Dynamics and Quality Control
- PMID: 28409163
- PMCID: PMC5376939
- DOI: 10.1155/2017/6483572
PGC1 α Activators Mitigate Diabetic Tubulopathy by Improving Mitochondrial Dynamics and Quality Control
Abstract
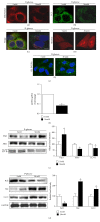
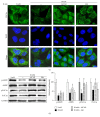
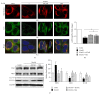
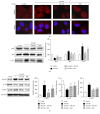
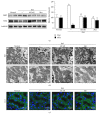
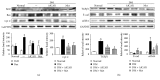
Purpose. In this study, we investigated the effect of PGC1α activators on mitochondrial fusion, fission, and autophagic quality control in renal tubular cells in a diabetic environment in vivo and in vitro. We also examined whether the upregulation of PGC1α attenuates diabetic tubulopathy by normalizing mitochondrial homeostasis. Methods. HKC8 cells were subjected to high-glucose conditions (30 mM D-glucose). Diabetes was induced with streptozotocin (STZ, 50 mg/kg i.p. for 5 days) in male C57/BL6J mice. AICAR or metformin was used as a PGC1α activator. Results. Treatment with the PGC1α activators AICAR and metformin improved functional mitochondrial mass in HKC8 cells in high-glucose conditions. Moreover, in renal proximal tubular cells, increased PGC1α activity correlated with the reversal of changes in Drp1, Mfn1, and LC3-II protein expression in a high-glucose environment. Normalized mitochondrial life cycles resulted in low ROS production and reduced apoptosis. AICAR and metformin treatment effectively mitigated albuminuria and renal histopathology and decreased the expression of TGFβ1 and αSMA in the kidneys of diabetic mice. Conclusions. Our results demonstrate that increases in PGC1α activity improve diabetic tubulopathy by modulating mitochondrial dynamics and autophagy.
Conflict of interest statement
All the authors declared no competing interests.
Figures


References
-
- Roglic G. WHO Global report on diabetes: a summary. International Journal of Noncommunicable Diseases. 2016;1(1):3–8. doi: 10.4103/2468-8827.184853. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

