A sphingosine-1-phosphate receptor type 1 agonist, ASP4058, suppresses intracranial aneurysm through promoting endothelial integrity and blocking macrophage transmigration
- PMID: 28409823
- PMCID: PMC5466536
- DOI: 10.1111/bph.13820
A sphingosine-1-phosphate receptor type 1 agonist, ASP4058, suppresses intracranial aneurysm through promoting endothelial integrity and blocking macrophage transmigration
Abstract
Background and purpose: Intracranial aneurysm (IA), common in the general public, causes lethal subarachnoid haemorrhage on rupture. It is, therefore, of utmost importance to prevent the IA from rupturing. However, there is currently no medical treatment. Recent studies suggest that IA is the result of chronic inflammation in the arterial wall caused by endothelial dysfunction and infiltrating macrophages. The sphingosine-1-phosphate receptor type 1 (S1P1 receptor) is present on the endothelium and promotes its barrier function. Here we have tested the potential of an S1P1 agonist, ASP4058, to prevent IA in an animal model.
Experimental approach: The effects of a selective S1P1 agonist, ASP4058, on endothelial permeability and migration of macrophages across an endothelial cell monolayer were tested in vitro using a Transwell system, and its effects on the size of IAs were evaluated in a rat model of IA.
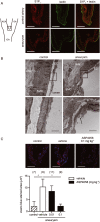
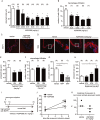
Key results: S1P1 receptor was expressed in endothelial cells of human IA lesions and control arterial walls. ASP4058 significantly reduced FITC-dextran leakage through an endothelial monolayer and suppressed the migration of macrophages across the monolayer in vitro. Oral administration of ASP4058 reduced the vascular permeability, macrophage infiltration and size of the IAs by acting as an S1P1 agonist in the rat model. This effect was mimicked by another two structurally-unrelated S1P1 agonists.
Conclusion and implications: A selective S1P1 agonist is a strong drug candidate for IA treatment as it promotes the endothelial cell barrier and suppresses the trans-endothelial migration of macrophages in IA lesions.
© 2017 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures



References
-
- Aoki T (2015). Inflammation mediates the pathogenesis of cerebral aneurysm and becomes therapeutic target. Neuroimmunol Neuroinflamm 2: 86–92.
-
- Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N (2009). Impact of monocyte chemoattractant protein‐1 deficiency on cerebral aneurysm formation. Stroke 40: 942–951. - PubMed
-
- Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T et al. (2007). NF‐kappaB is a key mediator of cerebral aneurysm formation. Circulation 116: 2830–2840. - PubMed
-
- Aoki T, Narumiya S (2012). Prostaglandins and chronic inflammation. Trends Pharmacol Sci 33: 304–311. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

