Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines
- PMID: 28410369
- PMCID: PMC5406007
- DOI: 10.1371/journal.pntd.0005544
Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines
Abstract
Background: Mass drug administration (MDA) of praziquantel has been the intervention of choice against schistosomiasis but with limited success in interrupting the transmission. The development of anti-Schistosoma vaccines is underway. Our objective is to quantify the population-level impact of anti-Schistosoma vaccines when administered alone and in combination with mass drug administration (MDA) and determine factors in vaccine design and public health implementation that optimize vaccination role in schistosomiasis control and elimination.
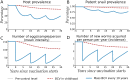
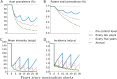
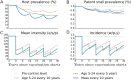
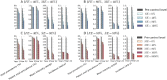
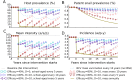
Methods and findings: We developed a deterministic compartmental model simulation of schistosomiasis transmission in a high-risk Kenyan community, including stratification by age, parasite burden, and vaccination status. The modeled schistosomiasis vaccines differed in terms of vaccine duration of protection (durability) and three biological efficacies. These are vaccine susceptibility effect (SE) of reducing person's susceptibility to Schistosoma acquisition, vaccine mortality effect (ME) of reducing established worm burden and vaccine fecundity effect (FE) of reducing egg release by mature worms. We quantified the population-level impact of vaccination over two decades under diverse vaccination schemes (childhood vs. mass campaigns), with different age-targeting scenarios, different risk settings, and with combined intervention with MDA. We also assessed the sensitivity of our predictions to uncertainties in model parameters. Over two decades, our base case vaccine with 80% SE, FE, and ME efficacies, 10 years' durability, provided by mass vaccination every 10 years, reduced host prevalence, mean intensity, incidence, and patent snail prevalence to 31%, 20 eggs/10-ml sample/person, 0.87 worm/person-year, and 0.74%, from endemic-state values of 71%, 152, 3.3, and 0.98%, respectively. Lower impact was found when coverage did not encompass all potential contaminators, and childhood-only vaccination schemes showed delayed and lower impact. In lower prevalence settings, the base case vaccine generated a proportionately smaller impact. A substantially larger vaccine program effect was generated when MDA + mass vaccination was provided every 5 years, which could be achieved by an MDA-only program only if drug was offered annually. Vaccine impact on schistosomiasis transmission was sensitive to a number of parameters including vaccine efficacies, human contact rates with water, human density, patent snails' rate of patency and lifespan, and force of infection to snails.
Conclusions: To be successful a vaccine-based control strategy will need a moderately to highly effective formulation combined with early vaccination of potential contaminators and aggressive coverage in repeated rounds of mass vaccination. Compared to MDA-only program, vaccination combined with MDA accelerates and prolongs the impact by reducing the acquisition of new worms and reducing egg release from residual worms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Modelling the impact of a Schistosoma mansoni vaccine and mass drug administration to achieve morbidity control and transmission elimination.PLoS Negl Trop Dis. 2019 Jun 5;13(6):e0007349. doi: 10.1371/journal.pntd.0007349. eCollection 2019 Jun. PLoS Negl Trop Dis. 2019. PMID: 31166956 Free PMC article.
-
Interrupting seasonal transmission of Schistosoma haematobium and control of soil-transmitted helminthiasis in northern and central Côte d'Ivoire: a SCORE study protocol.BMC Public Health. 2018 Jan 29;18(1):186. doi: 10.1186/s12889-018-5044-2. BMC Public Health. 2018. PMID: 29378542 Free PMC article. Clinical Trial.
-
Refined stratified-worm-burden models that incorporate specific biological features of human and snail hosts provide better estimates of Schistosoma diagnosis, transmission, and control.Parasit Vectors. 2016 Aug 4;9(1):428. doi: 10.1186/s13071-016-1681-4. Parasit Vectors. 2016. PMID: 27492409 Free PMC article.
-
Transmission control for schistosomiasis - why it matters now.Trends Parasitol. 2006 Dec;22(12):575-82. doi: 10.1016/j.pt.2006.09.006. Epub 2006 Oct 9. Trends Parasitol. 2006. PMID: 17030017 Review.
-
Schistosomiasis epidemiology and control: how did we get here and where should we go?Mem Inst Oswaldo Cruz. 2001;96 Suppl:17-27. doi: 10.1590/s0074-02762001000900003. Mem Inst Oswaldo Cruz. 2001. PMID: 11586422 Review.
Cited by
-
Control Strategies for Carcinogenic-Associated Helminthiases: An Integrated Overview.Front Cell Infect Microbiol. 2021 Mar 24;11:626672. doi: 10.3389/fcimb.2021.626672. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33842386 Free PMC article. Review.
-
Priority knowledge gaps for schistosomiasis research and development in the World Health Organization Africa Region.Infect Dis Poverty. 2025 Mar 17;14(1):19. doi: 10.1186/s40249-025-01285-w. Infect Dis Poverty. 2025. PMID: 40098025 Free PMC article.
-
Application of Schistosomiasis Consortium for Operational Research and Evaluation Study Findings to Refine Predictive Modeling of Schistosoma mansoni and Schistosoma haematobium Control in Sub-Saharan Africa.Am J Trop Med Hyg. 2020 Jul;103(1_Suppl):97-104. doi: 10.4269/ajtmh.19-0852. Am J Trop Med Hyg. 2020. PMID: 32400357 Free PMC article. Review.
-
Vaccination or mass drug administration against schistosomiasis: a hypothetical cost-effectiveness modelling comparison.Parasit Vectors. 2019 Oct 23;12(1):499. doi: 10.1186/s13071-019-3749-4. Parasit Vectors. 2019. PMID: 31647019 Free PMC article.
-
A comprehensive and critical overview of schistosomiasis vaccine candidates.J Parasit Dis. 2021 Jun;45(2):557-580. doi: 10.1007/s12639-021-01387-w. Epub 2021 Apr 25. J Parasit Dis. 2021. PMID: 33935395 Free PMC article. Review.
References
-
- WHO | Schistosomiasis. In: WHO [Internet]. [cited 22 Mar 2016]. http://www.who.int/mediacentre/factsheets/fs115/en/
-
- WHO | Preventive chemotherapy in human helminthiasis [Internet]. [cited 24 May 2016]. http://apps.who.int/iris/bitstream/10665/43545/1/9241547103_eng.pdf
-
- Study to Evaluate the Safety of the Vaccine Prepared sm14 Against Schistosomiasis—Full Text View—ClinicalTrials.gov [Internet]. [cited 23 Mar 2016]. https://clinicaltrials.gov/ct2/show/NCT01154049
-
- Efficacy of Vaccine Sh28GST in Association With Praziquantel (PZQ) for Prevention of Clinical Recurrences of Schistosoma Haematobium Pathology—Full Text View—ClinicalTrials.gov [Internet]. [cited 23 Mar 2016]. https://clinicaltrials.gov/ct2/show/NCT00870649
-
- A Phase I Study of the Safety, Reactogenicity, and Immunogenicity of Sm-TSP-2/Alhydrogel With or Without GLA-AF for Intestinal Schistosomiasis in Healthy Adults—Full Text View—ClinicalTrials.gov [Internet]. [cited 23 Mar 2016]. https://clinicaltrials.gov/ct2/show/NCT02337855
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

