Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer's disease: Alleviation by pantethine
- PMID: 28410378
- PMCID: PMC5391924
- DOI: 10.1371/journal.pone.0175369
Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer's disease: Alleviation by pantethine
Erratum in
-
Correction: Metabolic changes and inflammation in cultured astrocytes from the 5xFAD mouse model of Alzheimer's disease: Alleviation by pantethine.PLoS One. 2018 Mar 14;13(3):e0194586. doi: 10.1371/journal.pone.0194586. eCollection 2018. PLoS One. 2018. PMID: 29538465 Free PMC article.
Abstract
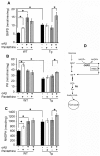
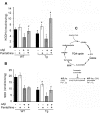
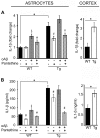
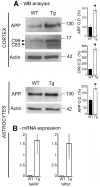
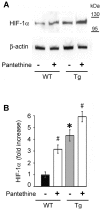
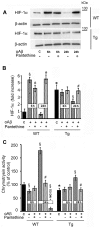
Astrocytes play critical roles in central nervous system homeostasis and support of neuronal function. A better knowledge of their response may both help understand the pathophysiology of Alzheimer's disease (AD) and implement new therapeutic strategies. We used the 5xFAD transgenic mouse model of AD (Tg thereafter) to generate astrocyte cultures and investigate the impact of the genotype on metabolic changes and astrocytes activation. Metabolomic analysis showed that Tg astrocytes exhibited changes in the glycolytic pathway and tricarboxylic acid (TCA) cycle, compared to wild type (WT) cells. Tg astrocytes displayed also a prominent basal inflammatory status, with accentuated reactivity and increased expression of the inflammatory cytokine interleukin-1 beta (IL-1β). Compensatory mechanisms were activated in Tg astrocytes, including: i) the hexose monophosphate shunt with the consequent production of reducing species; ii) the induction of hypoxia inducible factor-1 alpha (HIF-1α), known to protect against amyloid-β (Aβ) toxicity. Such events were associated with the expression by Tg astrocytes of human isoforms of both amyloid precursor protein (APP) and presenilin-1 (PS1). Similar metabolic and inflammatory changes were induced in WT astrocytes by exogenous Aβ peptide. Pantethine, the vitamin B5 precursor, known to be neuroprotective and anti-inflammatory, alleviated the pathological pattern in Tg astrocytes as well as WT astrocytes treated with Aß. In conclusion, our data enlighten the dual pathogenic/protective role of astrocytes in AD pathology and the potential protective role of pantethine.
Conflict of interest statement
Figures

References
-
- Kalman M (2004) Glia reaction and reactive glia In: Hertz L, editor. Non neuronal cells of the nervous system: function and dysfunction. Amsterdam: Elsevier; pp. 787–835.
-
- Hong HS, Hwang EM, Sim HJ, Cho HJ, Boo JH, Oh SS, et al. Interferon gamma stimulates beta-secretase expression and sAPPbeta production in astrocytes. Biochem Biophys Res Commun. 2003; 307:922–927. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

