Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: A systematic review of recently published studies
- PMID: 28410405
- PMCID: PMC5391942
- DOI: 10.1371/journal.pone.0175679
Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: A systematic review of recently published studies
Abstract
Most patients with advanced non-small cell lung cancer (NSCLC) have a poor prognosis and receive limited benefit from conventional treatments, especially in later lines of therapy. In recent years, several novel therapies have been approved for second- and third-line treatment of advanced NSCLC. In light of these approvals, it is valuable to understand the uptake of these new treatments in routine clinical practice and their impact on patient care. A systematic literature search was conducted in multiple scientific databases to identify observational cohort studies published between January 2010 and March 2017 that described second- or third-line treatment patterns and clinical outcomes in patients with advanced NSCLC. A qualitative data synthesis was performed because a meta-analysis was not possible due to the heterogeneity of the study populations. A total of 12 different study cohorts in 15 articles were identified. In these cohorts, single-agent chemotherapy was the most commonly administered treatment in both the second- and third-line settings. In the 5 studies that described survival from the time of second-line treatment initiation, median overall survival ranged from 4.6 months (95% CI, 3.8-5.7) to 12.8 months (95% CI, 10.7-14.5). There was limited information on the use of biomarker-directed therapy in these patient populations. This systematic literature review offers insights into the adoption of novel therapies into routine clinical practice for second- and third-line treatment of patients with advanced NSCLC. This information provides a valuable real-world context for the impact of recently approved treatments for advanced NSCLC.
Conflict of interest statement
Figures

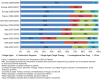
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; 2015. http://seer.cancer.gov/csr/1975_2012/.
-
- Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al.; CONCORD Working Group. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015; 385(9972):977–1010. 10.1016/S0140-6736(14)62038-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

