Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: A prospective randomized pilot study in healthy volunteers
- PMID: 28410406
- PMCID: PMC5391961
- DOI: 10.1371/journal.pone.0175626
Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: A prospective randomized pilot study in healthy volunteers
Abstract
Background and aims: The barrier function of the small intestinal mucosa prevents the introduction of undesired pathogens into the body. Breakdown of this barrier function increases intestinal permeability. This has been proposed to induce not only gastrointestinal diseases, including inflammatory bowel disease and irritable bowel syndrome, but also various other diseases, including allergies, diabetes mellitus, liver diseases, and collagen diseases, which are associated with this so called "leaky gut syndrome." As such, a method to prevent leaky gut syndrome would have substantial clinical value. However, no drugs have been demonstrated to improve disturbed intestinal permeability in humans to date. Therefore, we investigated whether a drug used to treat chronic constipation, lubiprostone, was effective for this purpose.
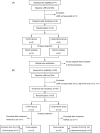
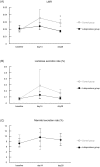
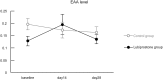
Methods: Healthy male volunteers were treated with lubiprostone (24 μg/day) for 28 days. Intestinal permeability was evaluated by measuring the lactulose-mannitol ratio (LMR) after administration of diclofenac and compared with an untreated group. The examination was conducted three times in total, i.e., at baseline before diclofenac administration and after 14 and 28 days of lubiprostone treatment. Blood endotoxin activity was also evaluated at the same time points.
Results: The final analysis was conducted on 28 subjects (14 in the lubiprostone group and 14 in the untreated group). The LMR after 28 days of treatment was significantly lower in the lubiprostone group than that in the untreated group (0.017 vs. 0.028, respectively; 95% confidence interval, -0.022--0.0001; p = 0.049). Blood endotoxin activity exhibited almost no change over time in the lubiprostone and untreated groups and displayed no significant differences at any time point of examination.
Conclusions: This study is the first to report an improvement in leaky gut using an available drug in humans. The result suggests that lubiprostone may prevent and ameliorate "leaky gut syndrome". However, a pivotal trial is needed to confirm our finding.
Conflict of interest statement
Figures

References
-
- Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18(5): 479–97. - PubMed
-
- Secondulfo M, Iafusco D, Carratù R, deMagistris L, Sapone A, Generoso M, et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig Liver Dis. 2004;36(1): 35–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

