Role of p16INK4a and BMI-1 in oxidative stress-induced premature senescence in human dental pulp stem cells
- PMID: 28410532
- PMCID: PMC5390672
- DOI: 10.1016/j.redox.2017.04.002
Role of p16INK4a and BMI-1 in oxidative stress-induced premature senescence in human dental pulp stem cells
Abstract
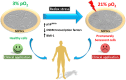
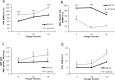
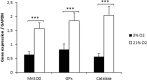
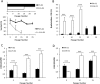
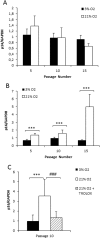
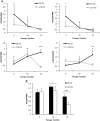
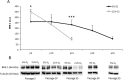
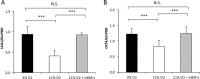
Human dental pulp stem cells (hDPSCs) are a source for cell therapy. Before implantation, an in vitro expansion step is necessary, with the inconvenience that hDPSCs undergo senescence following a certain number of passages, loosing their stemness properties. Long-term in vitro culture of hDPSCs at 21% (ambient oxygen tension) compared with 3-6% oxygen tension (physiological oxygen tension) caused an oxidative stress-related premature senescence, as evidenced by increased β-galactosidase activity and increased lysil oxidase expression, which is mediated by p16INK4a pathway. Furthermore, hDPSCs cultured at 21% oxygen tension underwent a downregulation of OCT4, SOX2, KLF4 and c-MYC factors, which was recued by BMI-1 silencing. Thus, p16INK4a and BMI-1 might play a role in the oxidative stress-associated premature senescence. We show that it is important for clinical applications to culture cells at physiological pO2 to retain their stemness characteristics and to delay senescence.
Keywords: Aging; Oxygen tension; Regenerative medicine.
Copyright © 2017. Published by Elsevier B.V.
Figures


Similar articles
-
p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response.Mech Ageing Dev. 2014 Nov-Dec;141-142:46-55. doi: 10.1016/j.mad.2014.09.004. Epub 2014 Oct 7. Mech Ageing Dev. 2014. PMID: 25304494
-
Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs).Cell Tissue Res. 2014 May;356(2):369-80. doi: 10.1007/s00441-014-1799-7. Epub 2014 Mar 28. Cell Tissue Res. 2014. PMID: 24676500
-
Bmi-1 reduction plays a key role in physiological and premature aging of primary human keratinocytes.J Invest Dermatol. 2010 Apr;130(4):1048-62. doi: 10.1038/jid.2009.355. Epub 2009 Nov 12. J Invest Dermatol. 2010. PMID: 19907431
-
Cyclin-dependent kinase inhibitor p16(INK4a) and telomerase may co-modulate endothelial progenitor cells senescence.Ageing Res Rev. 2008 Apr;7(2):137-46. doi: 10.1016/j.arr.2008.02.001. Epub 2008 Feb 12. Ageing Res Rev. 2008. PMID: 18343732 Review.
-
Cellular senescence in dental pulp stem cells.Arch Oral Biol. 2019 Mar;99:150-155. doi: 10.1016/j.archoralbio.2019.01.012. Epub 2019 Jan 17. Arch Oral Biol. 2019. PMID: 30685471 Review.
Cited by
-
Mesenchymal Stem Cell Senescence and Rejuvenation: Current Status and Challenges.Front Cell Dev Biol. 2020 Jun 3;8:364. doi: 10.3389/fcell.2020.00364. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582691 Free PMC article. Review.
-
Targeting Cell Senescence for the Treatment of Age-Related Bone Loss.Curr Osteoporos Rep. 2019 Apr;17(2):70-85. doi: 10.1007/s11914-019-00504-2. Curr Osteoporos Rep. 2019. PMID: 30806947 Review.
-
Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence.Stem Cell Res Ther. 2021 Feb 17;12(1):142. doi: 10.1186/s13287-021-02209-9. Stem Cell Res Ther. 2021. PMID: 33596998 Free PMC article.
-
PseudoCell: A Multivalued Logical Regulatory Network to Investigate Premature Senescence Dynamics and Heterogeneity.Aging Med (Milton). 2025 Apr 18;8(2):145-155. doi: 10.1002/agm2.70020. eCollection 2025 Apr. Aging Med (Milton). 2025. PMID: 40353054 Free PMC article.
-
Comparative analysis of cytokines and growth factors in the conditioned media of stem cells from the pulp of deciduous, young, and old permanent tooth.Saudi J Biol Sci. 2021 Jun;28(6):3559-3565. doi: 10.1016/j.sjbs.2021.03.031. Epub 2021 Mar 17. Saudi J Biol Sci. 2021. PMID: 34121899 Free PMC article.
References
-
- Mimeault M., Batra S.K. Aging of tissue-resident adult stem/progenitor cells and their pathological consequences. Panminerva Med. 2009;51:57–79. - PubMed
-
- Gu Y., Li T., Ding Y., Sun L., Tu T., Zhu W., Hu J., Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol. Med. Rep. 2016;13:5207–5215. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

