Evaluation of six serological ELISA kits available in Italy as screening tests for equine infectious anaemia surveillance
- PMID: 28410613
- PMCID: PMC5391595
- DOI: 10.1186/s12917-017-1007-6
Evaluation of six serological ELISA kits available in Italy as screening tests for equine infectious anaemia surveillance
Abstract
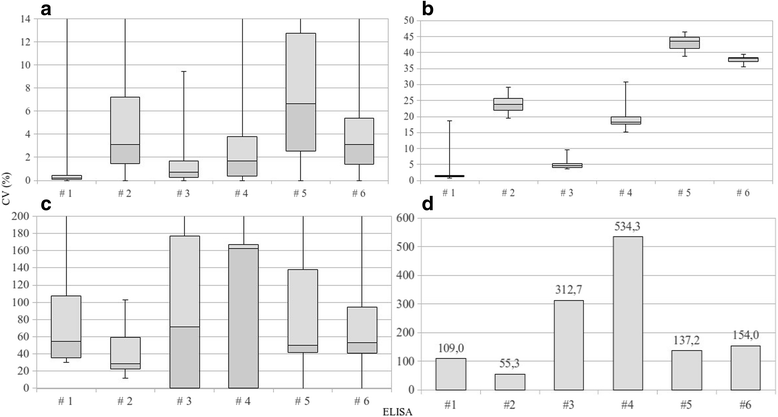
Background: ELISAs are known to have a higher diagnostic sensitivity than the agar gel immunodiffusion (AGID) when employed for serological diagnosis of equine infectious anaemia (EIA). For this purpose, an "in-house" and five commercial ELISAs available in Italy were assessed by the National Reference Centre for EIA for their analytic specificity (Sp); precocity, defined as capability of detecting first antibodies produced during a new infection; precision based on repeatability and reproducibility, estimated from the coefficient of variation (CV); accuracy, estimated from multiple K and relative Sp and sensitivity (Se). Two serum panels, positive for non-equine retroviruses and the most frequent equine viruses, were employed to measure analytic Sp. ELISA precocity was also compared to that of one "in-house" and three commercial AGID kits, employing a panel of sera, collected weekly from horses infected with modified EIA viruses. Precision and accuracy were defined using results of a panel containing positive and negative sera examined in an inter-laboratory trial with the participation of the ten Official Laboratories. Furthermore, a questionnaire was used to assess the appropriateness of each kit for routine use.
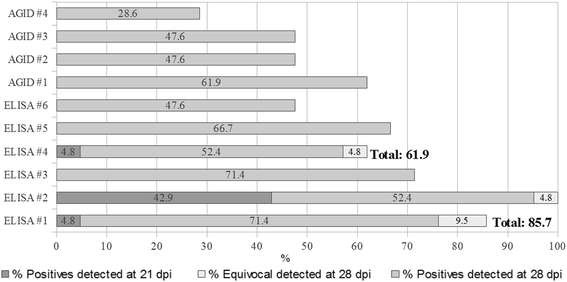
Results: Analytic Sp was 100%, while the 75th percentile of CVs for positive sera varied from 0.4% to 12.73% for repeatability and from 1.6% to 44.87% for reproducibility. Although CV of the negative serum was constantly high, its outcome was unaltered. Relative Se ranged from 98.2% to 100%, relative Sp was constantly 100% and multiple K ranged from 0.95 to 1. Precocity differed among the assays: three kits detected 4.8% and 42.9% positive samples on 21 days post infection (dpi), all assays detected positive samples on 28 dpi, between 47.6% and 95.2%. Precocity of ELISAs was superior to that of the AGIDs except for two assays. In view of the feedback obtained from the questionnaires, all kits were considered appropriate for routine use.
Conclusion: All ELISAs having high Se and precocity are preferable as a screening test in EIA surveillance programmes to the AGID tests examined. These two tests can be incorporated in a serial diagnostic pathway to improve the efficacy of a surveillance plan.
Keywords: AGID; Commercial assays; Comparison; ELISA; Equine infectious anaemia; In-house assays; Surveillance.
Figures
Similar articles
-
Comparison of commercial enzyme-linked immunosorbent assays and agar gel immunodiffusion tests for the serodiagnosis of equine infectious anemia.Can J Vet Res. 2004 Oct;68(4):254-8. Can J Vet Res. 2004. PMID: 15581219 Free PMC article.
-
Validation of an immunoblot assay employing an objective reading system and used as a confirmatory test in equine infectious anaemia surveillance programs.J Virol Methods. 2019 Apr;266:77-88. doi: 10.1016/j.jviromet.2019.01.012. Epub 2019 Jan 23. J Virol Methods. 2019. PMID: 30684508
-
Validation of an indirect ELISA employing a chimeric recombinant gag and env peptide for the serological diagnosis of equine infectious anemia.J Virol Methods. 2018 Jan;251:111-117. doi: 10.1016/j.jviromet.2017.10.002. Epub 2017 Oct 3. J Virol Methods. 2018. PMID: 28986292
-
[Equine Infectious Anemia (EIA)].Schweiz Arch Tierheilkd. 2009 Apr;151(4):159-64. doi: 10.1024/0036-7281.151.4.159. Schweiz Arch Tierheilkd. 2009. PMID: 19333901 Review. German.
-
Equine infectious anemia: prospects for control.Dev Biol Stand. 1990;72:49-57. Dev Biol Stand. 1990. PMID: 2178130 Review.
Cited by
-
Equine Infectious Anaemia: The Active Surveillance of an Entire Equid Population Reduces the Occurrence of the Infection.Transbound Emerg Dis. 2024 Apr 30;2024:3439871. doi: 10.1155/2024/3439871. eCollection 2024. Transbound Emerg Dis. 2024. PMID: 40303092 Free PMC article.
-
Comparative Evaluation of a Multistrain Indirect ELISA Targeting Anti- p26 and gp45 Antibodies for EIAV Detection.Pathogens. 2025 Jun 8;14(6):575. doi: 10.3390/pathogens14060575. Pathogens. 2025. PMID: 40559583 Free PMC article.
-
Identification and genetic characterization of equine infectious anemia virus in Western Balkans.BMC Vet Res. 2021 Apr 15;17(1):168. doi: 10.1186/s12917-021-02849-2. BMC Vet Res. 2021. PMID: 33858420 Free PMC article.
-
Serodiagnosis of equine infectious anemia by indirect ELISA based on a novel synthetic peptide derived from gp45 glycoprotein.Vet Res Commun. 2025 Apr 22;49(3):174. doi: 10.1007/s11259-025-10707-x. Vet Res Commun. 2025. PMID: 40261479 Free PMC article.
-
Development of a colloidal gold immunochromatographic strip to detect equine infectious anemia virus.Virol J. 2025 Jun 24;22(1):205. doi: 10.1186/s12985-025-02815-6. Virol J. 2025. PMID: 40555999 Free PMC article.
References
-
- Issel CJ, Cook SJ, Cook RF, Cordes TR. Optimal paradigms to detect reservoirs of equine infectious anemia virus (EIAV) J Equine Vet Sci. 1999;19:728–732. doi: 10.1016/S0737-0806(99)80140-3. - DOI
-
- World Organization For Animal Health: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2015. www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.05.06_EIA.pdf. Accessed 12 Feb 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

