Neutron reflectivity measurement of protein A-antibody complex at the solid-liquid interface
- PMID: 28410804
- PMCID: PMC5408906
- DOI: 10.1016/j.chroma.2017.03.084
Neutron reflectivity measurement of protein A-antibody complex at the solid-liquid interface
Abstract
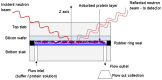
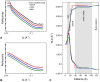
Chromatography is a ubiquitous unit operation in the purification of biopharmaceuticals yet few studies have addressed the biophysical characterisation of proteins at the solution-resin interface. Chromatography and other adsorption and desorption processes have been shown to induce protein aggregation which is undesirable in biopharmaceutical products. In order to advance understanding of how adsorption processes might impact protein stability, neutron reflectivity was used to characterise the structure of adsorbed immunoglobulin G (IgG) on model surfaces. In the first model system, IgG was adsorbed directly to silica and demonstrated a side-on orientation with high surface contact. A maximum dimension of 60Å in the surface normal direction and high density surface coverage were observed under pH 4.1 conditions. In chromatography buffers, pH was found to influence IgG packing density and orientation at the solid-liquid interface. In the second model system, which was designed to mimic an affinity chromatography surface, protein A was attached to a silica surface to produce a configuration representative of a porous glass chromatography resin. Interfacial structure was probed during sequential stages from ligand attachment, through to IgG binding and elution. Adsorbed IgG structures extended up to 250Å away from the surface and showed dependence on surface blocking strategies. The data was suggestive of two IgG molecules bound to protein A with a somewhat skewed orientation and close proximity to the silica surface. The findings provide insight into the orientation of adsorbed antibody structures under conditions encountered during chromatographic separations.
Keywords: Adsorption; Antibody; Chromatography; Interface; Neutron reflectivity; Protein A.
Copyright © 2017 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures








Similar articles
-
In situ neutron scattering of antibody adsorption during protein A chromatography.J Chromatogr A. 2020 Apr 26;1617:460842. doi: 10.1016/j.chroma.2019.460842. Epub 2020 Jan 9. J Chromatogr A. 2020. PMID: 31928770 Free PMC article.
-
Recent Advances in Studying Interfacial Adsorption of Bioengineered Monoclonal Antibodies.Molecules. 2020 Apr 28;25(9):2047. doi: 10.3390/molecules25092047. Molecules. 2020. PMID: 32353995 Free PMC article. Review.
-
Orientation of a monoclonal antibody adsorbed at the solid/solution interface: a combined study using atomic force microscopy and neutron reflectivity.Langmuir. 2006 Jul 4;22(14):6313-20. doi: 10.1021/la0532454. Langmuir. 2006. PMID: 16800692
-
Protein adsorption, desorption, and aggregation mediated by solid-liquid interfaces.J Pharm Sci. 2015 Jun;104(6):1946-1959. doi: 10.1002/jps.24429. Epub 2015 Apr 2. J Pharm Sci. 2015. PMID: 25846460
-
Adsorption properties of plant based bio-surfactants: Insights from neutron scattering techniques.Adv Colloid Interface Sci. 2019 Dec;274:102041. doi: 10.1016/j.cis.2019.102041. Epub 2019 Oct 17. Adv Colloid Interface Sci. 2019. PMID: 31655367 Review.
Cited by
-
In situ neutron scattering of antibody adsorption during protein A chromatography.J Chromatogr A. 2020 Apr 26;1617:460842. doi: 10.1016/j.chroma.2019.460842. Epub 2020 Jan 9. J Chromatogr A. 2020. PMID: 31928770 Free PMC article.
-
The pearl necklace model in protein A chromatography: Molecular mechanisms at the resin interface.Biotechnol Bioeng. 2019 Jan;116(1):76-86. doi: 10.1002/bit.26843. Epub 2018 Oct 25. Biotechnol Bioeng. 2019. PMID: 30252938 Free PMC article.
-
Adsorption of non-ionic surfactant and monoclonal antibody on siliconized surface studied by neutron reflectometry.J Colloid Interface Sci. 2021 Feb 15;584:429-438. doi: 10.1016/j.jcis.2020.09.110. Epub 2020 Oct 6. J Colloid Interface Sci. 2021. PMID: 33091867 Free PMC article.
-
A rapid and quantitative technique for assessing IgG monomeric purity, calibrated with the NISTmAb reference material.Anal Bioanal Chem. 2019 Sep;411(24):6487-6496. doi: 10.1007/s00216-019-02029-0. Epub 2019 Aug 2. Anal Bioanal Chem. 2019. PMID: 31375854 Free PMC article.
-
Recent Advances in Studying Interfacial Adsorption of Bioengineered Monoclonal Antibodies.Molecules. 2020 Apr 28;25(9):2047. doi: 10.3390/molecules25092047. Molecules. 2020. PMID: 32353995 Free PMC article. Review.
References
-
- Gagnon P., Nian R., Leong D., Hoi A. Transient conformational modification of immunoglobulin G during purification by protein A affinity chromatography. J. Chromatogr. A. 2015;1395:136–142. - PubMed
-
- Gagnon P., Nian R. Conformational plasticity of IgG during protein A affinity chromatography. J. Chromatogr. A. 2016;1433:98–105. - PubMed
-
- Pynn R. Neutron scattering—a non-destructive microscope for seeing inside matter. In: Liang L., Rinaldi R., Schober H., editors. Neutron Applications in Earth, Energy and Environmental Sciences. Springer; US: 2009.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

