Impact of Fabp1/Scp-2/Scp-x gene ablation (TKO) on hepatic phytol metabolism in mice
- PMID: 28411199
- PMCID: PMC5454513
- DOI: 10.1194/jlr.M075457
Impact of Fabp1/Scp-2/Scp-x gene ablation (TKO) on hepatic phytol metabolism in mice
Abstract
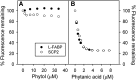
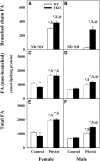
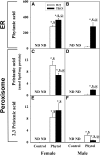
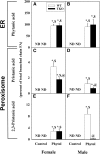
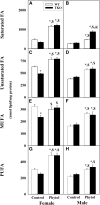
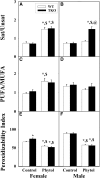
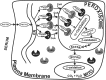
Studies in vitro have suggested that both sterol carrier protein-2/sterol carrier protein-x (Scp-2/Scp-x) and liver fatty acid binding protein [Fabp1 (L-FABP)] gene products facilitate hepatic uptake and metabolism of lipotoxic dietary phytol. However, interpretation of physiological function in mice singly gene ablated in the Scp-2/Scp-x has been complicated by concomitant upregulation of FABP1. The work presented herein provides several novel insights: i) An 8-anilino-1-naphthalenesulfonic acid displacement assay showed that neither SCP-2 nor L-FABP bound phytol, but both had high affinity for its metabolite, phytanic acid; ii) GC-MS studies with phytol-fed WT and Fabp1/Scp-2/SCP-x gene ablated [triple KO (TKO)] mice showed that TKO exacerbated hepatic accumulation of phytol metabolites in vivo in females and less so in males. Concomitantly, dietary phytol increased hepatic levels of total long-chain fatty acids (LCFAs) in both male and female WT and TKO mice. Moreover, in both WT and TKO female mice, dietary phytol increased hepatic ratios of saturated/unsaturated and polyunsaturated/monounsaturated LCFAs, while decreasing the peroxidizability index. However, in male mice, dietary phytol selectively increased the saturated/unsaturated ratio only in TKO mice, while decreasing the peroxidizability index in both WT and TKO mice. These findings suggested that: 1) SCP-2 and FABP1 both facilitated phytol metabolism after its conversion to phytanic acid; and 2) SCP-2/SCP-x had a greater impact on hepatic phytol metabolism than FABP1.
Keywords: fatty acid binding protein 1/sterol carrier protein-2/sterol carrier protein-x; peroxisomal oxidation; triple knockout.
Copyright © 2017 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Sex-dependent impact of Scp-2/Scp-x gene ablation on hepatic phytol metabolism.Arch Biochem Biophys. 2017 Dec 1;635:17-26. doi: 10.1016/j.abb.2017.10.011. Epub 2017 Oct 16. Arch Biochem Biophys. 2017. PMID: 29051070 Free PMC article.
-
Effect of Fabp1/Scp-2/Scp-x Ablation on Whole Body and Hepatic Phenotype of Phytol-Fed Male Mice.Lipids. 2017 May;52(5):385-397. doi: 10.1007/s11745-017-4249-y. Epub 2017 Apr 5. Lipids. 2017. PMID: 28382456 Free PMC article.
-
Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene.Am J Physiol Cell Physiol. 2005 Mar;288(3):C543-58. doi: 10.1152/ajpcell.00359.2004. Am J Physiol Cell Physiol. 2005. PMID: 15692150
-
Liver fatty acid-binding protein and obesity.J Nutr Biochem. 2010 Nov;21(11):1015-32. doi: 10.1016/j.jnutbio.2010.01.005. J Nutr Biochem. 2010. PMID: 20537520 Free PMC article. Review.
-
Phytanic acid: production from phytol, its breakdown and role in human disease.Cell Mol Life Sci. 2006 Aug;63(15):1752-65. doi: 10.1007/s00018-005-5463-y. Cell Mol Life Sci. 2006. PMID: 16799769 Free PMC article. Review.
Cited by
-
Sex-dependent impact of Scp-2/Scp-x gene ablation on hepatic phytol metabolism.Arch Biochem Biophys. 2017 Dec 1;635:17-26. doi: 10.1016/j.abb.2017.10.011. Epub 2017 Oct 16. Arch Biochem Biophys. 2017. PMID: 29051070 Free PMC article.
-
SCP2 variant is associated with alterations in lipid metabolism, brainstem neurodegeneration, and testicular defects.Hum Genomics. 2022 Aug 22;16(1):32. doi: 10.1186/s40246-022-00408-w. Hum Genomics. 2022. PMID: 35996156 Free PMC article.
-
Ablating both Fabp1 and Scp2/Scpx (TKO) induces hepatic phospholipid and cholesterol accumulation in high fat-fed mice.Biochim Biophys Acta Mol Cell Biol Lipids. 2018 Mar;1863(3):323-338. doi: 10.1016/j.bbalip.2017.12.013. Epub 2018 Jan 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2018. PMID: 29307784 Free PMC article.
-
Loss of fatty acid binding protein-1 alters the hepatic endocannabinoid system response to a high-fat diet.J Lipid Res. 2017 Nov;58(11):2114-2126. doi: 10.1194/jlr.M077891. Epub 2017 Oct 2. J Lipid Res. 2017. PMID: 28972119 Free PMC article.
-
Effect of Fabp1/Scp-2/Scp-x Ablation on Whole Body and Hepatic Phenotype of Phytol-Fed Male Mice.Lipids. 2017 May;52(5):385-397. doi: 10.1007/s11745-017-4249-y. Epub 2017 Apr 5. Lipids. 2017. PMID: 28382456 Free PMC article.
References
-
- Verhoeven N. M., and Jakobs C.. 2001. Human metabolism of phytanic acid and pristanic acid. Prog. Lipid Res. 40: 453–466. - PubMed
-
- Jansen G. A., and Wanders R. J. A.. 2006. Alpha oxidation. Biochim. Biophys. Acta. 1763: 1403–1412. - PubMed
-
- Steinberg D. 1978. Phytanic acid storage disease: Refsum’s syndrome In The Metabolic Basis of Inherited Disease. Stanbury J. B., Wyngaarden J. B., and Fredrickson D. S., editors. McGraw-Hill, New York: 688–706.
-
- Steinberg D. 1995. Refsums disease. In The Metabolic and Molecular Basis of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., et al., editors. McGraw-Hill, New York: 2351–2370.
-
- Gloerich J., van der Brink D. M., Ruiter J. P. N., van Vlies N., Vaz F. M., Wanders R. J. A., and Ferdinandusse S.. 2007. Metabolism of phytol to phytanic acid in the mouse, and the role of PPARalpha in its regulation. J. Lipid Res. 48: 77–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

