2-Alkylquinolone alkaloid biosynthesis in the medicinal plant Evodia rutaecarpa involves collaboration of two novel type III polyketide synthases
- PMID: 28411241
- PMCID: PMC5454096
- DOI: 10.1074/jbc.M117.778977
2-Alkylquinolone alkaloid biosynthesis in the medicinal plant Evodia rutaecarpa involves collaboration of two novel type III polyketide synthases
Abstract
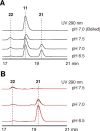
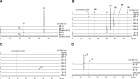
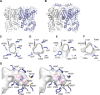
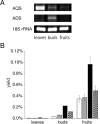
2-Alkylquinolone (2AQ) alkaloids are pharmaceutically and biologically important natural products produced by both bacteria and plants, with a wide range of biological effects, including antibacterial, cytotoxic, anticholinesterase, and quorum-sensing signaling activities. These diverse activities and 2AQ occurrence in vastly different phyla have raised much interest in the biosynthesis pathways leading to their production. Previous studies in plants have suggested that type III polyketide synthases (PKSs) might be involved in 2AQ biosynthesis, but this hypothesis is untested. To this end, we cloned two novel type III PKSs, alkyldiketide-CoA synthase (ADS) and alkylquinolone synthase (AQS), from the 2AQ-producing medicinal plant, Evodia rutaecarpa (Rutaceae). Functional analyses revealed that collaboration of ADS and AQS produces 2AQ via condensations of N-methylanthraniloyl-CoA, a fatty acyl-CoA, with malonyl-CoA. We show that ADS efficiently catalyzes the decarboxylative condensation of malonyl-CoA with a fatty acyl-CoA to produce an alkyldiketide-CoA, whereas AQS specifically catalyzes the decarboxylative condensation of an alkyldiketide acid with N-methylanthraniloyl-CoA to generate the 2AQ scaffold via C-C/C-N bond formations. Remarkably, the ADS and AQS crystal structures at 1.80 and 2.20 Å resolutions, respectively, indicated that the unique active-site architecture with Trp-332 and Cys-191 and the novel CoA-binding tunnel with Tyr-215 principally control the substrate and product specificities of ADS and AQS, respectively. These results provide additional insights into the catalytic versatility of the type III PKSs and their functional and evolutionary implications for 2AQ biosynthesis in plants and bacteria.
Keywords: Evodia rutaecarpa; alkaloid; alkyldiketide synthase; alkylquinolone synthase; biosynthesis; crystal structure; enzyme; polyketide; secondary metabolism; type III polyketide synthase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures









References
-
- Wang X. X., Zan K., Shi S. P., Zeng K. W., Jiang Y., Guan Y., Xiao C. L., Gao H. Y., Wu L. J., and Tu P. F. (2013) Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia 89, 1–7 - PubMed
-
- Wang C. F., Fan L., Tian M., Qi X. S., Liu J. X., Feng J. B., Du S. S., Su X., and Wang Y. Y. (2014) Radiosensitizing effect of schinifoline from Zanthoxylum schinifolium Sieb et Zucc on human non-small cell lung cancer A549 cells: a preliminary in vitro investigation. Molecules 19, 20128–20138 - PMC - PubMed
-
- Dulcey C. E., Dekimpe V., Fauvelle D. A., Milot S., Groleau M. C., Doucet N., Rahme L. G., Lépine F., and Déziel E. (2013) The end of an old hypothesis: the Pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem. Biol. 20, 1481–1491 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

