Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug-Drug Interaction Between Clopidogrel and Dasabuvir
- PMID: 28411400
- PMCID: PMC5599937
- DOI: 10.1002/cpt.689
Physiologically Based Pharmacokinetic Modeling Suggests Limited Drug-Drug Interaction Between Clopidogrel and Dasabuvir
Abstract
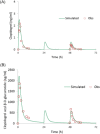
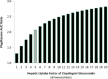
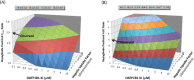
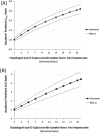
Dasabuvir, a nonnucleoside NS5B polymerase inhibitor, is a sensitive substrate of cytochrome P450 (CYP) 2C8 with a potential for drug-drug interaction (DDI) with clopidogrel. A physiologically based pharmacokinetic (PBPK) model was developed for dasabuvir to evaluate the DDI potential with clopidogrel, the acyl-β-D glucuronide metabolite of which has been reported as a strong mechanism-based inhibitor of CYP2C8 based on an interaction with repaglinide. In addition, the PBPK model for clopidogrel and its metabolite were updated with additional in vitro data. Sensitivity analyses using these PBPK models suggested that CYP2C8 inhibition by clopidogrel acyl-β-D glucuronide may not be as potent as previously suggested. The dasabuvir and updated clopidogrel PBPK models predict a moderate increase of 1.5-1.9-fold for Cmax and 1.9-2.8-fold for AUC of dasabuvir when coadministered with clopidogrel. While the PBPK results suggest there is a potential for DDI between dasabuvir and clopidogrel, the magnitude is not expected to be clinically relevant.
© 2017 The Authors Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

Similar articles
-
Utilizing PBPK Modeling to Evaluate the Potential of a Significant Drug-Drug Interaction Between Clopidogrel and Dasabuvir: A Scientific Perspective.Clin Pharmacol Ther. 2017 Oct;102(4):578-580. doi: 10.1002/cpt.699. Epub 2017 Apr 26. Clin Pharmacol Ther. 2017. PMID: 28444890
-
Glucuronidation converts clopidogrel to a strong time-dependent inhibitor of CYP2C8: a phase II metabolite as a perpetrator of drug-drug interactions.Clin Pharmacol Ther. 2014 Oct;96(4):498-507. doi: 10.1038/clpt.2014.141. Epub 2014 Jun 27. Clin Pharmacol Ther. 2014. PMID: 24971633 Clinical Trial.
-
Clarification of the Mechanism of Clopidogrel-Mediated Drug-Drug Interaction in a Clinical Cassette Small-dose Study and Its Prediction Based on In Vitro Information.Drug Metab Dispos. 2016 Oct;44(10):1622-32. doi: 10.1124/dmd.116.070276. Epub 2016 Jul 25. Drug Metab Dispos. 2016. PMID: 27457785
-
Inhibition of CYP2C8 by Acyl Glucuronides of Gemfibrozil and Clopidogrel: Pharmacological Significance, Progress and Challenges.Biomolecules. 2022 Sep 1;12(9):1218. doi: 10.3390/biom12091218. Biomolecules. 2022. PMID: 36139056 Free PMC article. Review.
-
Clinical Pharmacokinetics of Dasabuvir.Clin Pharmacokinet. 2017 Oct;56(10):1115-1124. doi: 10.1007/s40262-017-0519-3. Clin Pharmacokinet. 2017. PMID: 28258380 Review.
Cited by
-
Utilization of Physiologically Based Pharmacokinetic Modeling in Clinical Pharmacology and Therapeutics: an Overview.Curr Pharmacol Rep. 2020;6(3):71-84. doi: 10.1007/s40495-020-00212-x. Epub 2020 May 12. Curr Pharmacol Rep. 2020. PMID: 32399388 Free PMC article. Review.
-
In Vitro-In Silico Approach in the Development of Clopidogrel Solid Dispersion Formulations.Bioengineering (Basel). 2025 Mar 30;12(4):357. doi: 10.3390/bioengineering12040357. Bioengineering (Basel). 2025. PMID: 40281717 Free PMC article.
-
Interaction of Dasabuvir With Clopidogrel: Did Predictions by Physiologically Based Pharmacokinetics Modeling Pass the Test?Clin Pharmacol Ther. 2019 Feb;105(2):320-321. doi: 10.1002/cpt.1196. Epub 2018 Sep 17. Clin Pharmacol Ther. 2019. PMID: 30225892 Free PMC article. No abstract available.
-
Approaches to Dose Finding in Neonates, Illustrating the Variability between Neonatal Drug Development Programs.Pharmaceutics. 2020 Jul 20;12(7):685. doi: 10.3390/pharmaceutics12070685. Pharmaceutics. 2020. PMID: 32698409 Free PMC article.
-
A physiologically based pharmacokinetic model of clopidogrel in populations of European and Japanese ancestry: An evaluation of CYP2C19 activity.Pharmacol Res Perspect. 2022 Apr;10(2):e00946. doi: 10.1002/prp2.946. Pharmacol Res Perspect. 2022. PMID: 35307978 Free PMC article.
References
-
- Feld, J.J. et al Treatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. N. Engl. J. Med. 370, 1594–1603 (2014). - PubMed
-
- Ferenci, P. et al ABT‐450/r‐ombitasvir and dasabuvir with or without ribavirin for HCV. N. Engl. J. Med. 370, 1983–1992 (2014). - PubMed
-
- Shebley, M. et al Mechanisms and predictions of drug‐drug interactions of the hepatitis C virus 3‐direct acting antiviral (3D) regimen: paritaprevir/ritonavir, ombitasvir and dasabuvir. Drug Metab. Dispos. (2017). DOI:10.1124/dmd.116.074518 [Epub ahead of print]. - DOI - PubMed
-
- Viekira Pak (ombitasvir, paritaprevir, and ritonavir tablets; dasabuvir tablets) [US package insert]. North Chicago, IL; AbbVie, 2016. - PubMed
-
- Plavix (clopidogrel) [US package insert]. Bridgewater, NJ; Sanofi Aventis, 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

