Pregestational type 2 diabetes mellitus induces cardiac hypertrophy in the murine embryo through cardiac remodeling and fibrosis
- PMID: 28412087
- PMCID: PMC5787338
- DOI: 10.1016/j.ajog.2017.04.008
Pregestational type 2 diabetes mellitus induces cardiac hypertrophy in the murine embryo through cardiac remodeling and fibrosis
Abstract
Background: Cardiac hypertrophy is highly prevalent in patients with type 2 diabetes mellitus. Experimental evidence has implied that pregnant women with type 2 diabetes mellitus and their children are at an increased risk of cardiovascular diseases. Our previous mouse model study revealed that maternal type 2 diabetes mellitus induces structural heart defects in their offspring.
Objective: This study aims to determine whether maternal type 2 diabetes mellitus induces embryonic heart hypertrophy in a murine model of diabetic embryopathy.
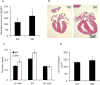
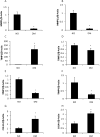
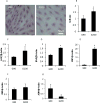
Study design: The type 2 diabetes mellitus embryopathy model was established by feeding 4-week-old female C57BL/6J mice with a high-fat diet for 15 weeks. Cardiac hypertrophy in embryos at embryonic day 17.5 was characterized by measuring heart size and thickness of the right and left ventricle walls and the interventricular septum, as well as the expression of β-myosin heavy chain, atrial natriuretic peptide, insulin-like growth factor-1, desmin, and adrenomedullin. Cardiac remodeling was determined by collagen synthesis and fibronectin synthesis. Fibrosis was evaluated by Masson staining and determining the expression of connective tissue growth factor, osteopontin, and galectin-3 genes. Cell apoptosis also was measured in the developing heart.
Results: The thicknesses of the left ventricle walls and the interventricular septum of embryonic hearts exposed to maternal diabetes were significantly thicker than those in the nondiabetic group. Maternal diabetes significantly increased β-myosin heavy chain, atrial natriuretic peptide, insulin-like growth factor-1, and desmin expression, but decreased expression of adrenomedullin. Moreover, collagen synthesis was significantly elevated, whereas fibronectin synthesis was suppressed, in embryonic hearts from diabetic dams, suggesting that cardiac remodeling is a contributing factor to cardiac hypertrophy. The cardiac fibrosis marker, galectin-3, was induced by maternal diabetes. Furthermore, maternal type 2 diabetes mellitus activated the proapoptotic c-Jun-N-terminal kinase 1/2 stress signaling and triggered cell apoptosis by increasing the number of terminal deoxynucleotidyl transferase 2'-deoxyuridine 5'-triphosphate nick end labeling-positive cells (10.4 ± 2.2% of the type 2 diabetes mellitus group vs 3.8 ± 0.7% of the nondiabetic group, P < .05).
Conclusion: Maternal type 2 diabetes mellitus induces cardiac hypertrophy in embryonic hearts. Adverse cardiac remodeling, including elevated collagen synthesis, suppressed fibronectin synthesis, profibrosis, and apoptosis, is implicated as the etiology of cardiac hypertrophy.
Keywords: cardiac remodeling; diabetic embryopathy; fibrosis; hypertrophy; pregestational type 2 diabetes.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




Similar articles
-
Type 2 diabetes mellitus induces congenital heart defects in murine embryos by increasing oxidative stress, endoplasmic reticulum stress, and apoptosis.Am J Obstet Gynecol. 2016 Sep;215(3):366.e1-366.e10. doi: 10.1016/j.ajog.2016.03.036. Epub 2016 Mar 31. Am J Obstet Gynecol. 2016. PMID: 27038779 Free PMC article.
-
Effects of gestational and pregestational diabetes mellitus on the foetal heart: a cross-sectional study.J Obstet Gynaecol. 2018 Apr;38(3):408-412. doi: 10.1080/01443615.2017.1410536. Epub 2018 Jan 21. J Obstet Gynaecol. 2018. PMID: 29355062
-
Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy.Am J Obstet Gynecol. 2008 Jan;198(1):130.e1-7. doi: 10.1016/j.ajog.2007.06.070. Am J Obstet Gynecol. 2008. PMID: 18166327
-
Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor.Am J Physiol Cell Physiol. 2009 Dec;297(6):C1490-500. doi: 10.1152/ajpcell.00049.2009. Epub 2009 Jul 22. Am J Physiol Cell Physiol. 2009. PMID: 19625611
-
Diabetes and Early Development: Epigenetics, Biological Stress, and Aging.Am J Perinatol. 2025 Jun;42(8):977-987. doi: 10.1055/a-2405-1493. Epub 2024 Aug 29. Am J Perinatol. 2025. PMID: 39209306 Review.
Cited by
-
Small Molecule Activators of Mitochondrial Fusion Prevent Congenital Heart Defects Induced by Maternal Diabetes.JACC Basic Transl Sci. 2024 Feb 14;9(3):303-318. doi: 10.1016/j.jacbts.2023.11.008. eCollection 2024 Mar. JACC Basic Transl Sci. 2024. PMID: 38559623 Free PMC article.
-
Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies.Children (Basel). 2025 Feb 28;12(3):312. doi: 10.3390/children12030312. Children (Basel). 2025. PMID: 40150594 Free PMC article.
-
Quantifying fetal heart health in gestational diabetes: a new approach with fetal heart quantification technology.Front Pharmacol. 2024 May 28;15:1394885. doi: 10.3389/fphar.2024.1394885. eCollection 2024. Front Pharmacol. 2024. PMID: 38863981 Free PMC article.
-
Changes in the shape and function of the fetal heart of pre- and gestational diabetes mothers.BMC Pregnancy Childbirth. 2024 Jan 11;24(1):57. doi: 10.1186/s12884-024-06262-z. BMC Pregnancy Childbirth. 2024. PMID: 38212679 Free PMC article.
-
Programming of cardiometabolic health: the role of maternal and fetal hyperinsulinaemia.J Endocrinol. 2022 Apr 5;253(2):R47-R63. doi: 10.1530/JOE-21-0332. J Endocrinol. 2022. PMID: 35258482 Free PMC article. Review.
References
-
- Blackmore HL, Ozanne SE. Programming of cardiovascular disease across the life-course. J Mol Cell Cardiol. 2015;83:122–30. - PubMed
-
- Dearden L, Ozanne SE. Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol. 2015;39:3–16. - PubMed
-
- Nicholas LM, Morrison JL, Rattanatray L, Zhang S, Ozanne SE, McMillen IC. The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes (Lond) 2016;40:229–38. - PubMed
-
- Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol. 2011;32:205–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

