Memantine improves outcomes after repetitive traumatic brain injury
- PMID: 28412305
- PMCID: PMC5640468
- DOI: 10.1016/j.bbr.2017.04.017
Memantine improves outcomes after repetitive traumatic brain injury
Abstract
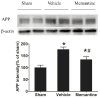
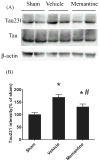
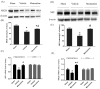
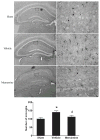
Repetitive mild traumatic brain injury (rmTBI; e.g., sports concussions) is common and results in significant cognitive impairment. Targeted therapies for rmTBI are lacking, though evidence from other injury models indicates that targeting N-methyl-d-aspartate (NMDA) receptor (NMDAR)-mediated glutamatergic toxicity might mitigate rmTBI-induced neurologic deficits. However, there is a paucity of preclinical or clinical data regarding NMDAR antagonist efficacy in the rmTBI setting. To test whether NMDAR antagonist therapy improves outcomes after rmTBI, mice were subjected to rmTBI injury (4 injuries in 4days) and randomized to treatment with the NMDA antagonist memantine or with vehicle. Functional outcomes were assessed by motor, anxiety/impulsivity and mnemonic behavioral tests. At the synaptic level, NMDAR-dependent long-term potentiation (LTP) was assessed in isolated neocortical slices. At the molecular level, the magnitude of gliosis and tau hyper-phosphorylation was tested by Western blot and immunostaining, and NMDAR subunit expression was evaluated by Western blot and polymerase chain reaction (PCR). Compared to vehicle-treated mice, memantine-treated mice had reduced tau phosphorylation at acute time points after injury, and less glial activation and LTP deficit 1 month after injury. Treatment with memantine also corresponded to normal NMDAR expression after rmTBI. No corresponding protection in behavior outcomes was observed. Here we found NMDAR antagonist therapy may improve histopathological and functional outcomes after rmTBI, though without consistent corresponding improvement in behavioral outcomes. These data raise prospects for therapeutic post-concussive NMDAR antagonism, particularly in athletes and warriors, who suffer functional impairment and neurodegenerative sequelae after multiple concussions.
Keywords: Concussion; NMDAR; Repetitive concussion; Traumatic brain injury.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Memantine Mitigates Oligodendrocyte Damage after Repetitive Mild Traumatic Brain Injury.Neuroscience. 2019 Nov 21;421:152-161. doi: 10.1016/j.neuroscience.2019.10.016. Epub 2019 Nov 1. Neuroscience. 2019. PMID: 31682950
-
Time-Dependent Long-Term Effect of Memantine following Repetitive Mild Traumatic Brain Injury.J Neurotrauma. 2024 Jul;41(13-14):e1736-e1758. doi: 10.1089/neu.2023.0423. Epub 2024 May 15. J Neurotrauma. 2024. PMID: 38666723
-
Environmental Enrichment Mitigates Deficits after Repetitive Mild Traumatic Brain Injury.J Neurotrauma. 2017 Aug 15;34(16):2445-2455. doi: 10.1089/neu.2016.4823. Epub 2017 Jun 8. J Neurotrauma. 2017. PMID: 28376667 Free PMC article.
-
The chemical biology of clinically tolerated NMDA receptor antagonists.J Neurochem. 2006 Jun;97(6):1611-26. doi: 10.1111/j.1471-4159.2006.03991.x. J Neurochem. 2006. PMID: 16805772 Review.
-
Why do many NMDA antagonists fail, while others are safe and effective at blocking excitotoxicity associated with dementia and acute injury?Am J Alzheimers Dis Other Demen. 2004 Sep-Oct;19(5):269-74. doi: 10.1177/153331750401900502. Am J Alzheimers Dis Other Demen. 2004. PMID: 15553982 Free PMC article. Review.
Cited by
-
Novel therapies for combating chronic neuropathological sequelae of TBI.Neuropharmacology. 2019 Feb;145(Pt B):160-176. doi: 10.1016/j.neuropharm.2018.06.021. Epub 2018 Jun 20. Neuropharmacology. 2019. PMID: 29933008 Free PMC article. Review.
-
Single Neuron Modeling Identifies Potassium Channel Modulation as Potential Target for Repetitive Head Impacts.Neuroinformatics. 2023 Jul;21(3):501-516. doi: 10.1007/s12021-023-09633-7. Epub 2023 Jun 9. Neuroinformatics. 2023. PMID: 37294503 Free PMC article.
-
Visual Dysfunction after Repetitive Mild Traumatic Brain Injury in a Mouse Model and Ramifications on Behavioral Metrics.J Neurotrauma. 2021 Oct 15;38(20):2881-2895. doi: 10.1089/neu.2021.0165. Epub 2021 Sep 2. J Neurotrauma. 2021. PMID: 34375128 Free PMC article.
-
Huperzine A alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury.Metab Brain Dis. 2017 Dec;32(6):1861-1869. doi: 10.1007/s11011-017-0075-4. Epub 2017 Jul 26. Metab Brain Dis. 2017. PMID: 28748496
-
The effects of mild closed head injuries on tauopathy and cognitive deficits in rodents: Primary results in wild type and rTg4510 mice, and a systematic review.Exp Neurol. 2020 Apr;326:113180. doi: 10.1016/j.expneurol.2020.113180. Epub 2020 Jan 11. Exp Neurol. 2020. PMID: 31930992 Free PMC article.
References
-
- Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychological medicine. 1973;3:270–303. - PubMed
-
- Martland H. Punch drunk. Journal of the American medical Association. 1928;91:1103–1107.
-
- Takahashi H, Manaka S, Sano K. Changes in extracellular potassium concentration in cortex and brain stem during the acute phase of experimental closed head injury. Journal of neurosurgery. 1981;55:708–717. - PubMed
-
- Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. Journal of neurosurgery. 1990;73:889–900. - PubMed
-
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? The Lancet. Neurology. 2002;1:383–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

