Chemical toxicity prediction for major classes of industrial chemicals: Is it possible to develop universal models covering cosmetics, drugs, and pesticides?
- PMID: 28412406
- PMCID: PMC5638676
- DOI: 10.1016/j.fct.2017.04.008
Chemical toxicity prediction for major classes of industrial chemicals: Is it possible to develop universal models covering cosmetics, drugs, and pesticides?
Abstract
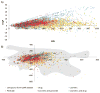
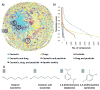
Computational models have earned broad acceptance for assessing chemical toxicity during early stages of drug discovery or environmental safety assessment. The majority of publicly available QSAR toxicity models have been developed for datasets including mostly drugs or drug-like compounds. We have evaluated and compared chemical spaces occupied by cosmetics, drugs, and pesticides, and explored whether current computational models of toxicity endpoints can be universally applied to all these chemicals. Our analysis of the chemical space overlap and applicability domain (AD) of models built previously for twenty different toxicity endpoints showed that most of these models afforded high coverage (>90%) for all three classes of compounds analyzed herein. Only T. pyriformis models demonstrated lower coverage for drugs and pesticides (38% and 54%, respectively). These results show that, for the most part, historical QSAR models built with data available for different toxicity endpoints can be used for toxicity assessment of novel chemicals irrespective of the intended commercial use; however, the AD restriction is necessary to assure the expected prediction accuracy. Local models may need to be developed to capture chemicals that appear as outliers with respect to global models.
Keywords: Chemical space; Cosmetics; Drugs; Pesticides; Prediction; QSAR models.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no actual or potential conflict of interests.
Figures


References
-
- Alves VM, Muratov EN, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, Tropsha A. Predicting chemically-induced skin reactions. Part I: QSAR models of skin sensitization and their application to identify potentially hazardous compounds. Toxicol Appl Pharmacol. 2015a;284:262–272. doi: 10.1016/j.taap.2014.12.014. - DOI - PMC - PubMed
-
- Alves VM, Muratov EN, Fourches D, Strickland J, Kleinstreuer N, Andrade CH, Tropsha A. Predicting chemically-induced skin reactions. Part II: QSAR models of skin permeability and the relationships between skin permeability and skin sensitization. Toxicol Appl Pharmacol. 2015b;284:273–280. doi: 10.1016/j.taap.2014.12.013. - DOI - PMC - PubMed
-
- Benfenati E, Benigni R, Demarini DM, Helma C, Kirkland D, Martin TM, Mazzatorta P, Ouédraogo-Arras G, Richard AM, Schilter B, Schoonen WGEJ, Snyder RD, Yang C. Predictive models for carcinogenicity and mutagenicity: frameworks, state-of-the-art, and perspectives. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:57–90. doi: 10.1080/10590500902885593. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

