Optogenetic stimulation of cortex to map evoked whisker movements in awake head-restrained mice
- PMID: 28412497
- PMCID: PMC5798595
- DOI: 10.1016/j.neuroscience.2017.04.004
Optogenetic stimulation of cortex to map evoked whisker movements in awake head-restrained mice
Abstract
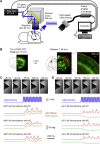
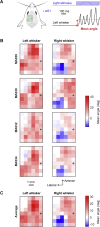
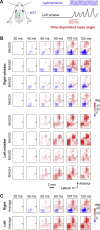
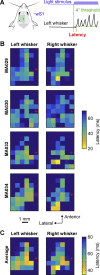
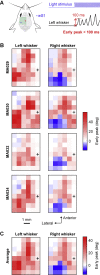
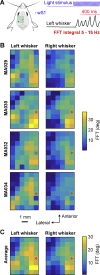
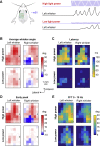
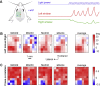
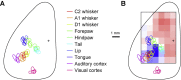
Whisker movements are used by rodents to touch objects in order to extract spatial and textural tactile information about their immediate surroundings. To understand the mechanisms of such active sensorimotor processing it is important to investigate whisker motor control. The activity of neurons in the neocortex affects whisker movements, but many aspects of the organization of cortical whisker motor control remain unknown. Here, we filmed whisker movements evoked by sequential optogenetic stimulation of different locations across the left dorsal sensorimotor cortex of awake head-restrained mice. Whisker movements were evoked by optogenetic stimulation of many regions in the dorsal sensorimotor cortex. Optogenetic stimulation of whisker sensory barrel cortex evoked retraction of the contralateral whisker after a short latency, and a delayed rhythmic protraction of the ipsilateral whisker. Optogenetic stimulation of frontal cortex evoked rhythmic bilateral whisker protraction with a longer latency compared to stimulation of sensory cortex. Compared to frontal cortex stimulation, larger amplitude bilateral rhythmic whisking in a less protracted position was evoked at a similar latency by stimulating a cortical region posterior to Bregma and close to the midline. These data suggest that whisker motor control might be broadly distributed across the dorsal mouse sensorimotor cortex. Future experiments must investigate the complex neuronal circuits connecting specific cell-types in various cortical regions with the whisker motor neurons located in the facial nucleus.
Keywords: barrel cortex; cortical motor map; motor cortex; optogenetics; sensory cortex; whisker motor control.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
Parallel pathways from motor and somatosensory cortex for controlling whisker movements in mice.Eur J Neurosci. 2015 Feb;41(3):354-67. doi: 10.1111/ejn.12800. Epub 2014 Dec 5. Eur J Neurosci. 2015. PMID: 25476605 Free PMC article.
-
Peripheral optogenetic stimulation induces whisker movement and sensory perception in head-fixed mice.Elife. 2016 Jun 8;5:e14140. doi: 10.7554/eLife.14140. Elife. 2016. PMID: 27269285 Free PMC article.
-
Behavioral Consequences of a Bifacial Map in the Mouse Somatosensory Cortex.J Neurosci. 2017 Jul 26;37(30):7209-7218. doi: 10.1523/JNEUROSCI.0598-17.2017. Epub 2017 Jun 29. J Neurosci. 2017. PMID: 28663199 Free PMC article.
-
Cortical control of whisker movement.Annu Rev Neurosci. 2014;37:183-203. doi: 10.1146/annurev-neuro-062012-170344. Epub 2014 May 9. Annu Rev Neurosci. 2014. PMID: 24821429 Review.
-
Sensorimotor processing in the rodent barrel cortex.Nat Rev Neurosci. 2019 Sep;20(9):533-546. doi: 10.1038/s41583-019-0200-y. Epub 2019 Jul 31. Nat Rev Neurosci. 2019. PMID: 31367018 Free PMC article. Review.
Cited by
-
Sparse and distributed cortical populations mediate sensorimotor integration.bioRxiv [Preprint]. 2023 Sep 22:2023.09.21.558857. doi: 10.1101/2023.09.21.558857. bioRxiv. 2023. PMID: 37790362 Free PMC article. Preprint.
-
Deficits in Behavioral Functions of Intact Barrel Cortex Following Lesions of Homotopic Contralateral Cortex.Front Syst Neurosci. 2018 Nov 22;12:57. doi: 10.3389/fnsys.2018.00057. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30524251 Free PMC article.
-
A spatially dynamic network underlies the generation of inspiratory behaviors.Proc Natl Acad Sci U S A. 2019 Apr 9;116(15):7493-7502. doi: 10.1073/pnas.1900523116. Epub 2019 Mar 27. Proc Natl Acad Sci U S A. 2019. PMID: 30918122 Free PMC article.
-
Sensory cortical control of movement.Nat Neurosci. 2020 Jan;23(1):75-84. doi: 10.1038/s41593-019-0536-7. Epub 2019 Nov 18. Nat Neurosci. 2020. PMID: 31740813
-
Cortico-Thalamo-Cortical Circuits of Mouse Forelimb S1 Are Organized Primarily as Recurrent Loops.J Neurosci. 2020 Apr 1;40(14):2849-2858. doi: 10.1523/JNEUROSCI.2277-19.2020. Epub 2020 Feb 19. J Neurosci. 2020. PMID: 32075900 Free PMC article.
References
-
- Akemann W., Mutoh H., Perron A., Rossier J., Knöpfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. - PubMed
-
- Ayling O.G., Harrison T.C., Boyd J.D., Goroshkov A., Murphy T.H. Automated light-based mapping of motor cortex by photoactivation of channelrhodopsin-2 transgenic mice. Nat Methods. 2009;6:219–224. - PubMed
-
- Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

