Opportunities for developing therapies for rare genetic diseases: focus on gain-of-function and allostery
- PMID: 28412959
- PMCID: PMC5392956
- DOI: 10.1186/s13023-017-0614-4
Opportunities for developing therapies for rare genetic diseases: focus on gain-of-function and allostery
Abstract
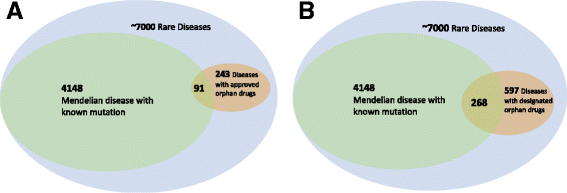
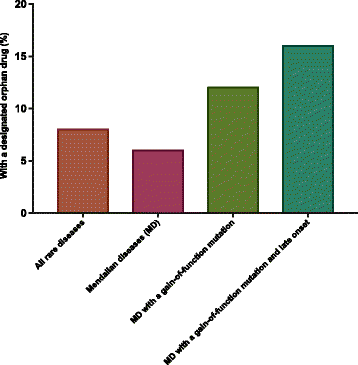
Background: Advances in next generation sequencing technologies have revolutionized our ability to discover the causes of rare genetic diseases. However, developing treatments for these diseases remains challenging. In fact, when we systematically analyze the US FDA orphan drug list, we find that only 8% of rare diseases have an FDA-designated drug. Our approach leverages three primary insights: first, diseases with gain-of-function mutations and late onset are more likely to have drug options; second, drugs are more often inhibitors than activators; and third, some disease-causing proteins can be rescued by allosteric activators in diseases due to loss-of-function mutations.
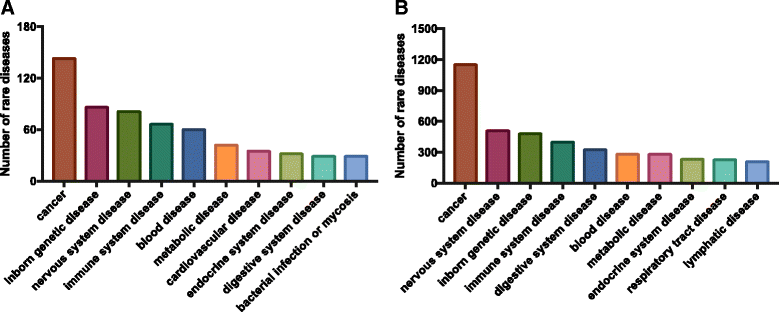
Results: We have developed a pipeline that combines natural language processing and human curation to mine promising targets for drug development from the Online Mendelian Inheritance in Man (OMIM) database. This pipeline targets diseases caused by well-characterized gain-of-function mutations or loss-of-function proteins with known allosteric activators. Applying this pipeline across thousands of rare genetic diseases, we discover 34 rare genetic diseases that are promising candidates for drug development.
Conclusion: Our analysis has revealed uneven coverage of rare diseases in the current US FDA orphan drug space. Diseases with gain-of-function mutations or loss-of-function mutations and known allosteric activators should be prioritized for drug treatments.
Keywords: Allosteric; Drug discovery; Drug targets; Gain-of-function; Genetic diseases; Orphan drugs; Rare diseases.
Figures

References
-
- Braun MM, Farag-El-Massah S, Xu K, Coté TR. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov. 2010;9(7):519–522. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

