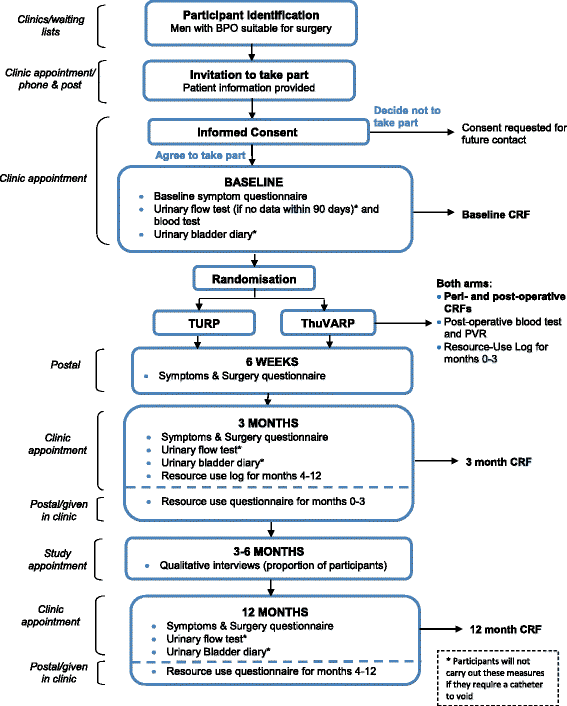
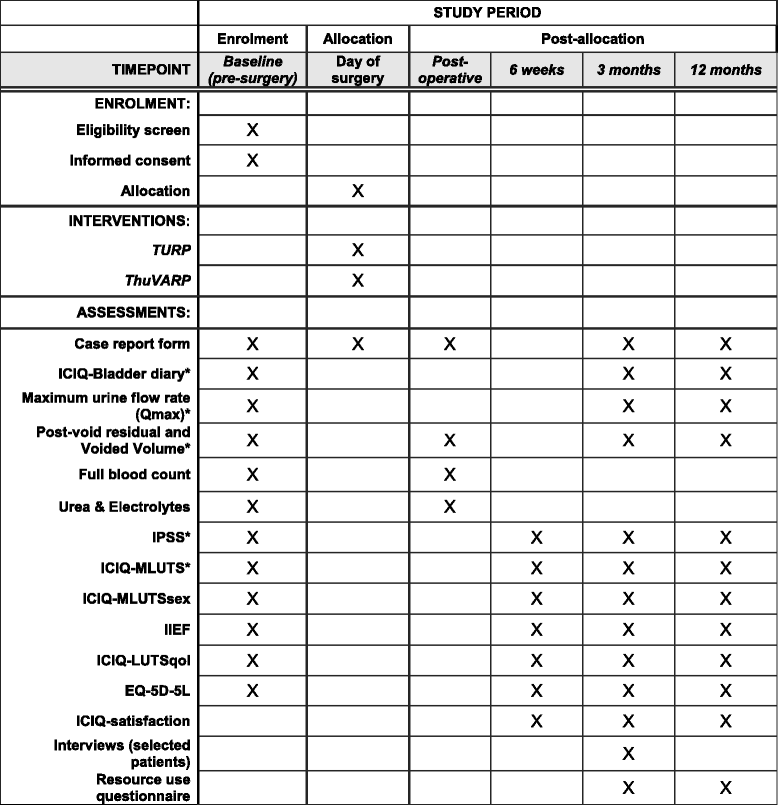
A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral vaporesection of the prostate (ThuVARP) versus transurethral resection of the prostate (TURP) in the National Health Service (NHS) - the UNBLOCS trial: a study protocol for a randomised controlled trial
- PMID: 28412960
- PMCID: PMC5392965
- DOI: 10.1186/s13063-017-1916-5
A randomised controlled trial to determine the clinical and cost effectiveness of thulium laser transurethral vaporesection of the prostate (ThuVARP) versus transurethral resection of the prostate (TURP) in the National Health Service (NHS) - the UNBLOCS trial: a study protocol for a randomised controlled trial
Abstract
Background: Transurethral resection of the prostate (TURP) has been the standard operation for benign prostatic obstruction (BPO) for 40 years, with approximately 25,000 procedures performed annually, and has remained largely unchanged. It is generally a successful operation, but has well-documented risks for the patient. Thulium laser transurethral vaporesection of the prostate (ThuVARP) vaporises and resects the prostate using a surgical technique similar to TURP. The small amount of study data currently available suggests that ThuVARP may have certain advantages over TURP, including reduced blood loss and shorter hospital stay, earlier return to normal activities, and shorter duration of catheterisation.
Design: A multicentre, pragmatic, randomised, controlled, parallel-group trial of ThuVARP versus standard TURP in men with BPO. Four hundred and ten men suitable for prostate surgery were randomised to receive either ThuVARP or TURP at four university teaching hospitals, and three district general hospitals. The key aim of the trial is to determine whether ThuVARP is equivalent to TURP judged on both the patient-reported International Prostate Symptom Score (IPSS) and the maximum urine flow rate (Qmax) at 12 months post-surgery.
Discussion: The general population has an increased life expectancy. As men get older their prostates enlarge, potentially causing BPO, which often requires surgery. Therefore, as the population ages, more prostate operations are needed to relieve obstruction. There is hence sustained interest in the condition and increasing need to find safer techniques than TURP. Various laser techniques have become available but none are widely used in the NHS because of lengthy training required for surgeons or inferior performance on clinical outcomes. Promising initial evidence from one RCT shows that ThuVARP has equivalent clinical effectiveness when compared to TURP, as well as other potential advantages. As ThuVARP uses a technique similar to that used in TURP, the learning curve is short, potentially making it also very quickly generalisable. This randomised study is designed to provide the high-quality evidence, in an NHS setting, with a range of patient-reported, clinical and cost-effectiveness outcomes, which will underpin and inform future NICE guidance.
Trial registration: ISRCTN registry, ISRCTN00788389 . Registered on 20 September 2013.
Keywords: Benign prostatic obstruction; Lower urinary tract symptoms; Prostate; Randomised controlled trial; Surgery; TURP; ThuVARP; Thulium laser transurethral vaporesection of the prostate; Transurethral resection of the prostate; UNBLOCS.
Figures
References
-
- National Clinical Guideline Centre: Acute and Chronic Conditions. Lower urinary tract symptoms: the management of lower urinary tract symptoms in men; NICE clinical guideline 97. National Institute for Health and Clinical Excellence site, 2010. Available at: https://www.nice.org.uk/guidance/cg97. Accessed Apr 2017.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials