Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells
- PMID: 28412969
- PMCID: PMC5392955
- DOI: 10.1186/s13287-017-0518-1
Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells
Abstract
Background: Glioblastoma (GBM), a highly aggressive brain tumor, contains a subpopulation of glioblastoma stem-like cells (GSCs) that play roles in tumor maintenance, invasion, and therapeutic resistance. GSCs are therefore a promising target for GBM treatment. Our group identified the cellular prion protein (PrPC) and its partner, the co-chaperone Hsp70/90 organizing protein (HOP), as potential target candidates due to their role in GBM tumorigenesis and in neural stem cell maintenance.
Methods: GSCs expressing different levels of PrPC were cultured as neurospheres with growth factors, and characterized with stem cells markers and adhesion molecules markers through immunofluorescence and flow cytometry. We than evaluated GSC self-renewal and proliferation by clonal density assays and BrdU incorporation, respectively, in front of recombinant HOP treatment, combined or not with a HOP peptide which mimics the PrPC binding site. Stable silencing of HOP was also performed in parental and/or PrPC-depleted cell populations, and proliferation in vitro and tumor growth in vivo were evaluated. Migration assays were performed on laminin-1 pre-coated glass.
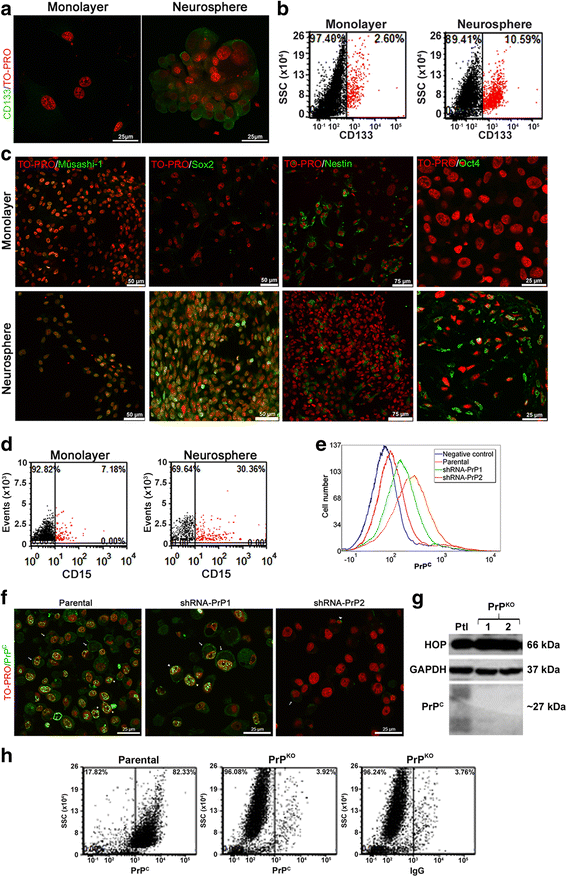
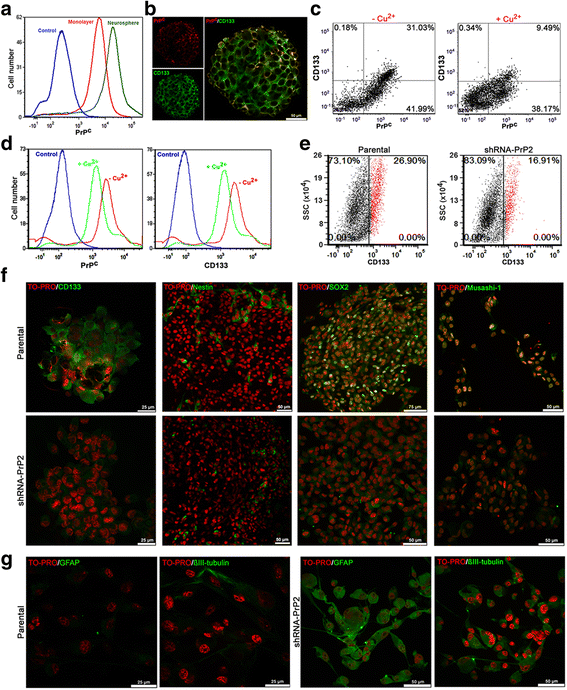
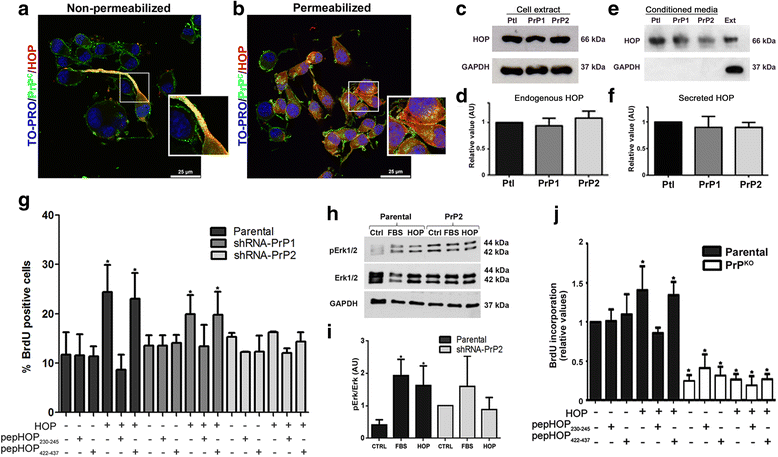
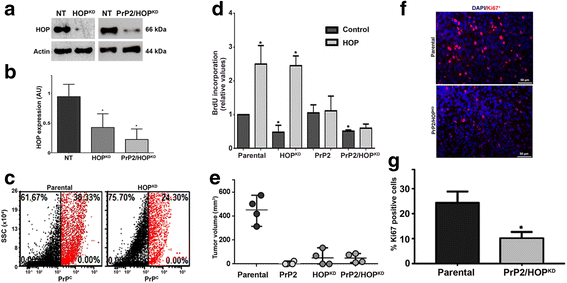
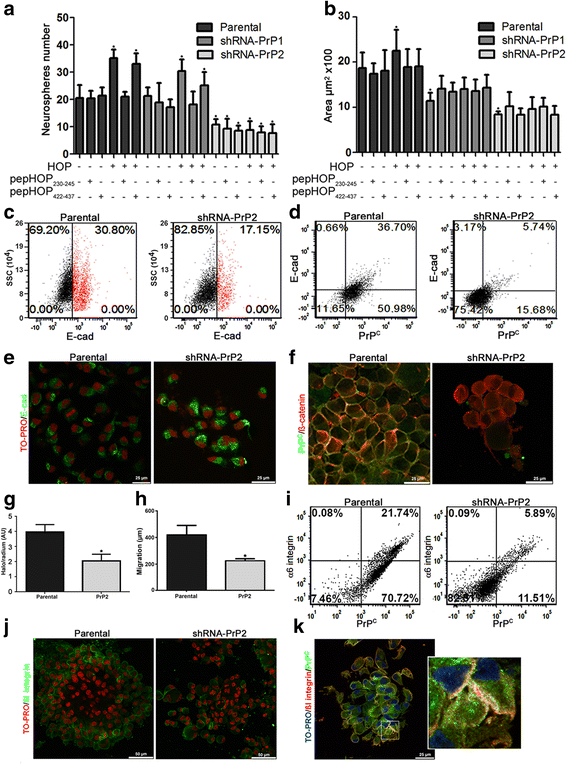
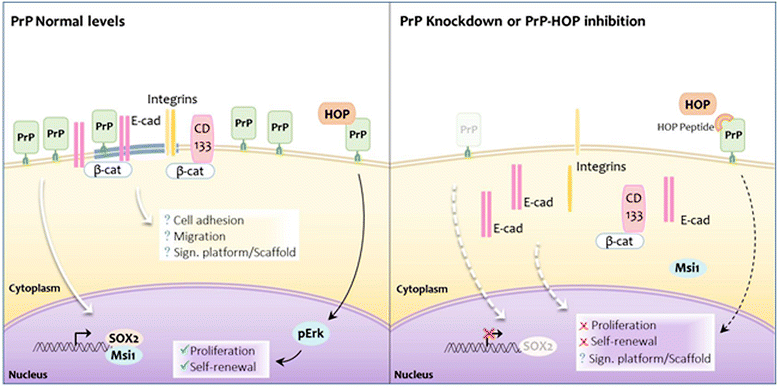
Results: We observed that, when GBM cells are cultured as neurospheres, they express specific stemness markers such as CD133, CD15, Oct4, and SOX2; PrPC is upregulated compared to monolayer culture and co-localizes with CD133. PrPC silencing downregulates the expression of molecules associated with cancer stem cells, upregulates markers of cell differentiation and affects GSC self-renewal, pointing to a pivotal role for PrPC in the maintenance of GSCs. Exogenous HOP treatment increases proliferation and self-renewal of GSCs in a PrPC-dependent manner while HOP knockdown disturbs the proliferation process. In vivo, PrPC and/or HOP knockdown potently inhibits the growth of subcutaneously implanted glioblastoma cells. In addition, disruption of the PrPC-HOP complex by a HOP peptide, which mimics the PrPC binding site, affects GSC self-renewal and proliferation indicating that the HOP-PrPC complex is required for GSC stemness. Furthermore, PrPC-depleted GSCs downregulate cell adhesion-related proteins and impair cell migration indicating a putative role for PrPC in the cell surface stability of cell adhesion molecules and GBM cell invasiveness, respectively.
Conclusions: In conclusion, our results show that the modulation of HOP-PrPC engagement or the decrease of PrPC and HOP expression may represent a potential therapeutic intervention in GBM, regulating glioblastoma stem-like cell self-renewal, proliferation, and migration.
Keywords: Cellular prion protein; Glioblastoma; Hsp70/90 organizing protein; Proliferation; Stem cells.
Figures
Similar articles
-
Prion protein regulates invasiveness in glioblastoma stem cells.BMC Cancer. 2024 Dec 18;24(1):1539. doi: 10.1186/s12885-024-13285-4. BMC Cancer. 2024. PMID: 39695426 Free PMC article.
-
Disruption of prion protein-HOP engagement impairs glioblastoma growth and cognitive decline and improves overall survival.Oncogene. 2015 Jun;34(25):3305-14. doi: 10.1038/onc.2014.261. Epub 2014 Aug 25. Oncogene. 2015. PMID: 25151961
-
Morusin inhibits glioblastoma stem cell growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation, and apoptosis induction.Mol Carcinog. 2016 Jan;55(1):77-89. doi: 10.1002/mc.22260. Epub 2014 Dec 31. Mol Carcinog. 2016. PMID: 25557841
-
Targeting prion protein interactions in cancer.Prion. 2015;9(3):165-73. doi: 10.1080/19336896.2015.1027855. Prion. 2015. PMID: 26110608 Free PMC article. Review.
-
Prion Protein in Glioblastoma Multiforme.Int J Mol Sci. 2019 Oct 15;20(20):5107. doi: 10.3390/ijms20205107. Int J Mol Sci. 2019. PMID: 31618844 Free PMC article. Review.
Cited by
-
Prion Protein Expression is Correlated with Glioma Grades.Virol Sin. 2020 Aug;35(4):490-493. doi: 10.1007/s12250-020-00209-2. Epub 2020 Mar 31. Virol Sin. 2020. PMID: 32236816 Free PMC article. No abstract available.
-
Glioma stem cells: drivers of tumor progression and recurrence.Stem Cell Res Ther. 2025 Jun 7;16(1):293. doi: 10.1186/s13287-025-04352-z. Stem Cell Res Ther. 2025. PMID: 40483525 Free PMC article. Review.
-
The multiple functions of PrPC in physiological, cancer, and neurodegenerative contexts.J Mol Med (Berl). 2022 Oct;100(10):1405-1425. doi: 10.1007/s00109-022-02245-9. Epub 2022 Sep 3. J Mol Med (Berl). 2022. PMID: 36056255 Review.
-
High cellular prion protein expression in cholangiocarcinoma: A marker for early postoperative recurrence and unfavorable prognosis.World J Gastrointest Surg. 2025 Mar 27;17(3):101940. doi: 10.4240/wjgs.v17.i3.101940. World J Gastrointest Surg. 2025. PMID: 40162420 Free PMC article.
-
Integrated transcriptomics uncovers an enhanced association between the prion protein gene expression and vesicle dynamics signatures in glioblastomas.BMC Cancer. 2024 Feb 13;24(1):199. doi: 10.1186/s12885-024-11914-6. BMC Cancer. 2024. PMID: 38347462 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

