Appraising the holistic value of Lenvatinib for radio-iodine refractory differentiated thyroid cancer: A multi-country study applying pragmatic MCDA
- PMID: 28412971
- PMCID: PMC5393009
- DOI: 10.1186/s12885-017-3258-9
Appraising the holistic value of Lenvatinib for radio-iodine refractory differentiated thyroid cancer: A multi-country study applying pragmatic MCDA
Abstract
Background: The objective of the study was to reveal through pragmatic MCDA (EVIDEM) the contribution of a broad range of criteria to the value of the orphan drug lenvatinib for radioiodine refractory differentiated thyroid cancer (RR-DTC) in country-specific contexts.
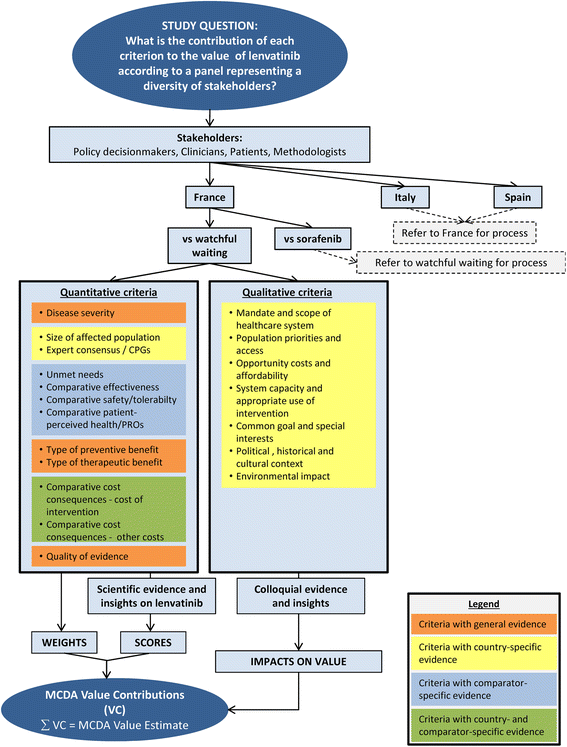
Methods: The study was designed to enable comprehensive appraisal (12 quantitative, 7 qualitative criteria) in the current disease context (watchful waiting, sorafenib) of France, Italy and Spain. Data on the value of lenvatinib was collected from diverse stakeholders during country-specific panels and included: criteria weights (individual and social values); performance scores (judgments on evidence-collected through MCDA systematic review); qualitative impacts of contextual criteria; and verbal and written insights structured by criteria. The value contribution of each criterion was calculated and uncertainty explored.
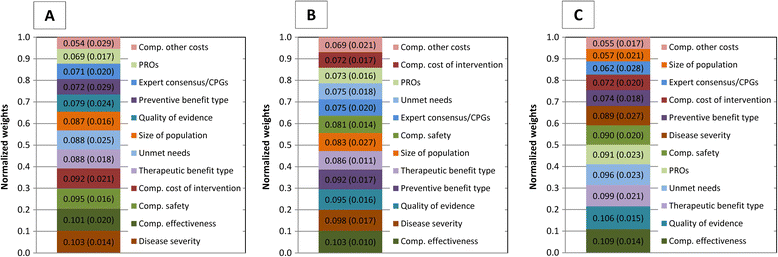
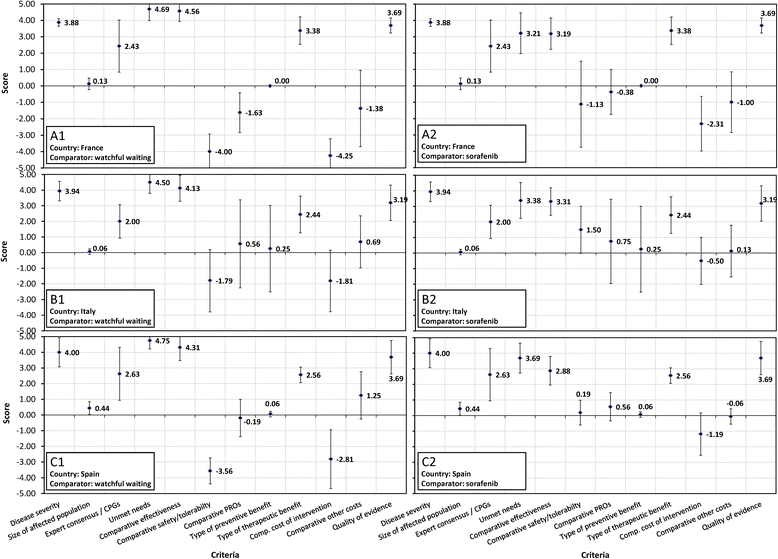
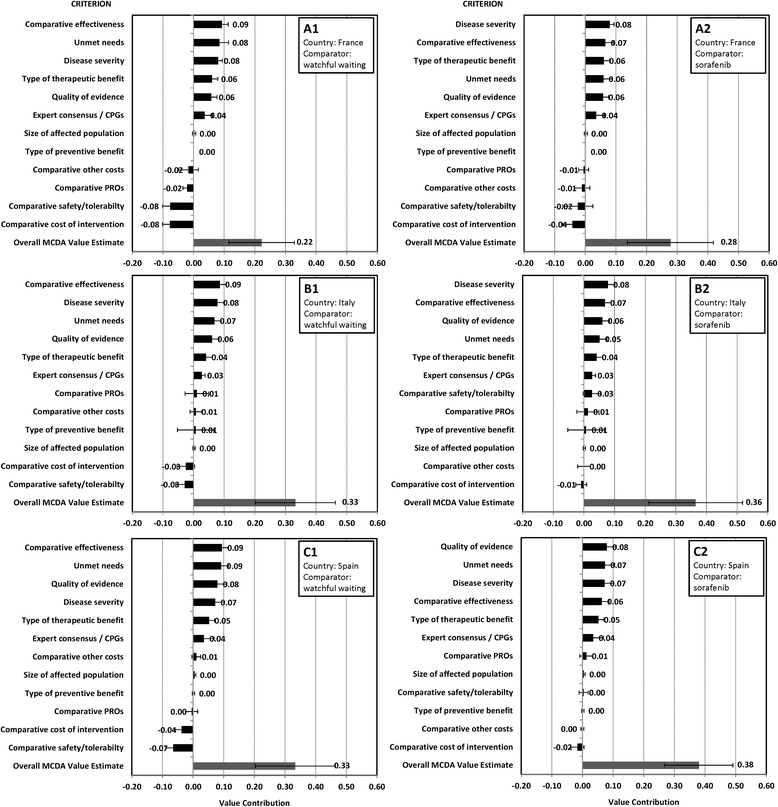
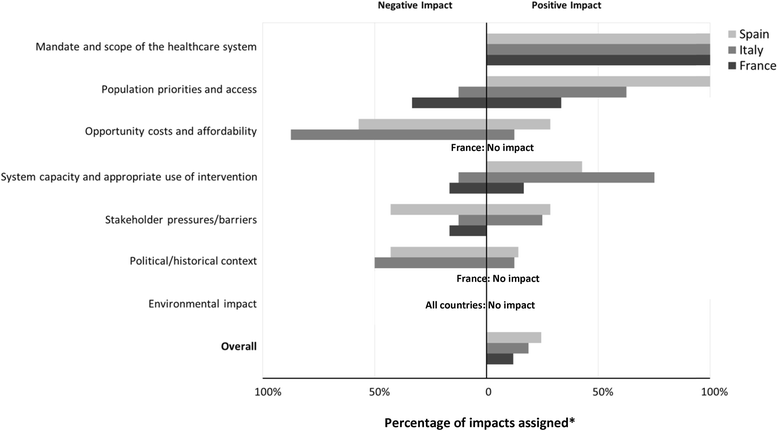
Results: Comparative effectiveness, Quality of evidence (Spain and Italy) and Disease severity (France) received the greatest weights. Four criteria contributed most to the value of lenvatinib, reflecting its superior Comparative effectiveness (16-22% of value), the severity of RR-DTC (16-22%), significant unmet needs (14-21%) and robust evidence (14-20%). Contributions varied by comparator, country and individuals, highlighting the importance of context and consultation. Results were reproducible at the group level. Impacts of contextual criteria varied across countries reflecting different health systems and cultural backgrounds. The MCDA process promoted sharing stakeholders' knowledge on lenvatinib and insights on context.
Conclusions: The value of lenvatinib was consistently positive across diverse therapeutic contexts. MCDA identified the aspects contributing most to value, revealed rich contextual insights, and helped participants express and explicitly tackle ethical trade-offs inherent to balanced appraisal and decisionmaking.
Keywords: Appraisal; Healthcare decisionmaking; Lenvatinib; Mcda.
Figures
References
-
- European Medicines Agency . Product characteristics. Lenvima. 2015.
-
- European Medicines Agency . CHMP assessment report. Lenvima. 2015.
-
- European Medicines Agency. Product information. Nexavar. On European Medicines Agency website. 2015. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed 26 Mar 2015.
-
- Tuttle RM, Haddad RI, Ball DW, Byrd D, Dickson P, Duh QY, et al. Thyroid carcinoma, version 2.2014. J Natl Compr Cancer Netw. 2014;12(12):1671–1680. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

