Biochemical and Biological Attributes of Matrix Metalloproteinases
- PMID: 28413025
- PMCID: PMC5430303
- DOI: 10.1016/bs.pmbts.2017.02.005
Biochemical and Biological Attributes of Matrix Metalloproteinases
Abstract
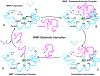
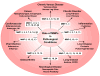
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that are involved in the degradation of various proteins in the extracellular matrix (ECM). Typically, MMPs have a propeptide sequence, a catalytic metalloproteinase domain with catalytic zinc, a hinge region or linker peptide, and a hemopexin domain. MMPs are commonly classified on the basis of their substrates and the organization of their structural domains into collagenases, gelatinases, stromelysins, matrilysins, membrane-type (MT)-MMPs, and other MMPs. MMPs are secreted by many cells including fibroblasts, vascular smooth muscle (VSM), and leukocytes. MMPs are regulated at the level of mRNA expression and by activation of their latent zymogen form. MMPs are often secreted as inactive pro-MMP form which is cleaved to the active form by various proteinases including other MMPs. MMPs cause degradation of ECM proteins such as collagen and elastin, but could influence endothelial cell function as well as VSM cell migration, proliferation, Ca2+ signaling, and contraction. MMPs play a role in tissue remodeling during various physiological processes such as angiogenesis, embryogenesis, morphogenesis, and wound repair, as well as in pathological conditions such as myocardial infarction, fibrotic disorders, osteoarthritis, and cancer. Increases in specific MMPs could play a role in arterial remodeling, aneurysm formation, venous dilation, and lower extremity venous disorders. MMPs also play a major role in leukocyte infiltration and tissue inflammation. MMPs have been detected in cancer, and elevated MMP levels have been associated with tumor progression and invasiveness. MMPs can be regulated by endogenous tissue inhibitors of metalloproteinases (TIMPs), and the MMP/TIMP ratio often determines the extent of ECM protein degradation and tissue remodeling. MMPs have been proposed as biomarkers for numerous pathological conditions and are being examined as potential therapeutic targets in various cardiovascular and musculoskeletal disorders as well as cancer.
Keywords: Cell signaling; Collagen; Extracellular matrix; Protein degradation; Proteinases; Remodeling.
© 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
None
Figures


References
-
- Mittal R, Patel AP, Debs LH, Nguyen D, Patel K, Grati M, Mittal J, Yan D, Chapagain P, Liu XZ. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. Journal of cellular physiology. 2016;231(12):2599–2621. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

