Investigation of OMNIgene·SPUTUM performance in delayed tuberculosis testing by smear, culture, and Xpert MTB/RIF assays in Uganda
- PMID: 28413105
- PMCID: PMC7320428
- DOI: 10.1016/j.jegh.2017.04.001
Investigation of OMNIgene·SPUTUM performance in delayed tuberculosis testing by smear, culture, and Xpert MTB/RIF assays in Uganda
Abstract
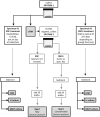
OMNIgene·SPUTUM (OM-S) is a sample transport reagent designed to work with all tuberculosis diagnostics while eliminating the need for cold chain. OM-S-treated sputum samples were assayed in several tests after multiday holds. Raw sputa from 100 patients underwent direct smear microscopy, were manually split and assigned to the OM-S group [OM-S added at collection (no other processing required) and tested after 0- to 5-day holds at room temperature] or standard-of-care (SOC) group (NaOH/N-acetyl l-cysteine decontamination, all tested on day of collection). Concentrated smear microscopy, Lowenstein Jensen (LJ) culture, and mycobacteria growth indicator tube (MGIT) culture were performed. For patients with negative direct smear, a second sample was split, with SOC (raw sputum) and OM-S portions (sediment) tested in the Xpert MTB/RIF (Xpert) assay. OM-S group and SOC group results were strongly concordant on all four tests [range, 89% (MGIT)-97% (Xpert)]. OM-S MGIT, LJ, and Xpert tests were in statistical agreement with SOC MGIT as reference. OM-S specimens had lower culture contamination rates (3% vs. 10% LJ; 2% vs. 5% MGIT) but required, on average, 5.6 additional days to become MGIT-positive. The findings suggest that samples held/transported in OM-S are compatible with smear microscopy, LJ or MGIT culture, and Xpert, and perform comparably to fresh sputum samples. Larger feasibility studies are warranted.
Keywords: MGIT; Molecular detection; Mycobacterium tuberculosis; Preservation; Solid culture; Specimen transport medium.
Copyright © 2017. Published by Elsevier Ltd.
Conflict of interest statement
CD Kelly-Cirino was employed by DNA Genotek during the execution of the study and the writing of the manuscript. PS Curry is employed by DNA Genotek.
Figures
Similar articles
-
Evaluation of OMNIgene Sputum and Ethanol Reagent for Preservation of Sputum Prior to Xpert and Culture Testing in Uganda.J Clin Microbiol. 2019 Dec 23;58(1):e00810-19. doi: 10.1128/JCM.00810-19. Print 2019 Dec 23. J Clin Microbiol. 2019. PMID: 31619525 Free PMC article.
-
Evaluation of OMNIgene®•SPUTUM-stabilised sputum for long-term transport and Xpert® MTB/RIF testing in Nepal.Int J Tuberc Lung Dis. 2016 Dec 1;20(12):1661-1667. doi: 10.5588/ijtld.16.0421. Int J Tuberc Lung Dis. 2016. PMID: 28000586
-
Diagnostic accuracy of Xpert MTB/RIF Ultra and culture assays to detect Mycobacterium Tuberculosis using OMNIgene-sputum processed stool among adult TB presumptive patients in Uganda.PLoS One. 2023 Apr 21;18(4):e0284041. doi: 10.1371/journal.pone.0284041. eCollection 2023. PLoS One. 2023. PMID: 37083706 Free PMC article.
-
Commercial products to preserve specimens for tuberculosis diagnosis: a systematic review.Int J Tuberc Lung Dis. 2018 Jul 1;22(7):741-753. doi: 10.5588/ijtld.17.0816. Int J Tuberc Lung Dis. 2018. PMID: 29914599
-
Systematic Review of Pooling Sputum as an Efficient Method for Xpert MTB/RIF Tuberculosis Testing during the COVID-19 Pandemic.Emerg Infect Dis. 2021 Mar;27(3):719-727. doi: 10.3201/eid2703.204090. Emerg Infect Dis. 2021. PMID: 33622482 Free PMC article.
Cited by
-
Evaluation of OMNIgene Sputum and Ethanol Reagent for Preservation of Sputum Prior to Xpert and Culture Testing in Uganda.J Clin Microbiol. 2019 Dec 23;58(1):e00810-19. doi: 10.1128/JCM.00810-19. Print 2019 Dec 23. J Clin Microbiol. 2019. PMID: 31619525 Free PMC article.
-
OMNIgene.SPUTUM suppresses contaminants while maintaining Mycobacterium tuberculosis viability and obviates cold-chain transport.ERJ Open Res. 2018 Feb 16;4(1):00074-2017. doi: 10.1183/23120541.00074-2017. eCollection 2018 Jan. ERJ Open Res. 2018. PMID: 29479537 Free PMC article.
-
Guidance for Studies Evaluating the Accuracy of Sputum-Based Tests to Diagnose Tuberculosis.J Infect Dis. 2019 Oct 8;220(220 Suppl 3):S99-S107. doi: 10.1093/infdis/jiz258. J Infect Dis. 2019. PMID: 31593597 Free PMC article.
-
Development and evaluation of novel bio-safe filter paper-based kits for sputum microscopy and transport to directly detect Mycobacterium tuberculosis and associated drug resistance.PLoS One. 2019 Aug 13;14(8):e0220967. doi: 10.1371/journal.pone.0220967. eCollection 2019. PLoS One. 2019. PMID: 31408508 Free PMC article. Clinical Trial.
-
Evaluation of Omnigene-Sputum for Preservation of Sputum Samples for Diagnosis of Mycobacterium tuberculosis.Trop Med Infect Dis. 2023 Jul 17;8(7):367. doi: 10.3390/tropicalmed8070367. Trop Med Infect Dis. 2023. PMID: 37505663 Free PMC article.
References
-
- Stop TB Partnership High-burden Countries Lists, World Health Organization. Available at: http://www.stoptb.org/countries/tbdata.asp. Accessed 02/09/2017.
-
- Stop TB Partnership Global Plan to End TB 2016–2020. Available at: http://www.stoptb.org/global/plan/plan2/. Accessed 02/09/2017.
-
- DNA Genotek Product Data Sheet PD-BR-00195. Available at: www.dnagenotek.com. Accessed 02/09/2017.
-
- DNA Genotek Product Data Sheet PD-BR-00196. Available at: www.dnagenotek.com. Accessed 02/09/2017.
-
- Niles J, Ray B, Curry P, Kelly-Cirino C. Compatibility of OMNIgene®·SPUTUM and prepIT®·MAX with molecular assays for tuberculosis: Real-time PCR and Hain Lifescience GenoType MTBC line probe assay. DNA Genotek Whitepaper PD-WP-00051. Available at: www.dnagenotek.com. Accessed 02/09/2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
