Rapid and coordinated processing of global motion images by local clusters of retinal ganglion cells
- PMID: 28413199
- PMCID: PMC5489431
- DOI: 10.2183/pjab.93.015
Rapid and coordinated processing of global motion images by local clusters of retinal ganglion cells
Erratum in
-
Errata to "Rapid and coordinated processing of global motion images by local clusters of retinal ganglion cells".Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):524. doi: 10.2183/pjab.93.033. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 28769022 Free PMC article. No abstract available.
Abstract
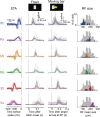
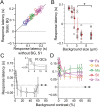
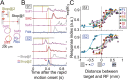
Even when the body is stationary, the whole retinal image is always in motion by fixational eye movements and saccades that move the eye between fixation points. Accumulating evidence indicates that the brain is equipped with specific mechanisms for compensating for the global motion induced by these eye movements. However, it is not yet fully understood how the retina processes global motion images during eye movements. Here we show that global motion images evoke novel coordinated firing in retinal ganglion cells (GCs). We simultaneously recorded the firing of GCs in the goldfish isolated retina using a multi-electrode array, and classified each GC based on the temporal profile of its receptive field (RF). A moving target that accompanied the global motion (simulating a saccade following a period of fixational eye movements) modulated the RF properties and evoked synchronized and correlated firing among local clusters of the specific GCs. Our findings provide a novel concept for retinal information processing during eye movements.
Figures






Similar articles
-
Global Jitter Motion of the Retinal Image Dynamically Alters the Receptive Field Properties of Retinal Ganglion Cells.Front Neurosci. 2019 Sep 13;13:979. doi: 10.3389/fnins.2019.00979. eCollection 2019. Front Neurosci. 2019. PMID: 31572123 Free PMC article.
-
Pharmacological properties of motion vision in goldfish measured with the optomotor response.Brain Res. 2005 Oct 5;1058(1-2):17-29. doi: 10.1016/j.brainres.2005.07.073. Epub 2005 Sep 16. Brain Res. 2005. PMID: 16150425
-
Non-linear retinal processing supports invariance during fixational eye movements.Vision Res. 2016 Jan;118:158-70. doi: 10.1016/j.visres.2015.10.012. Epub 2015 Nov 12. Vision Res. 2016. PMID: 26525844
-
A physiological perspective on fixational eye movements.Vision Res. 2016 Jan;118:31-47. doi: 10.1016/j.visres.2014.12.006. Epub 2014 Dec 20. Vision Res. 2016. PMID: 25536465 Free PMC article. Review.
-
Control and Functions of Fixational Eye Movements.Annu Rev Vis Sci. 2015 Nov;1:499-518. doi: 10.1146/annurev-vision-082114-035742. Epub 2015 Oct 14. Annu Rev Vis Sci. 2015. PMID: 27795997 Free PMC article. Review.
Cited by
-
Global Jitter Motion of the Retinal Image Dynamically Alters the Receptive Field Properties of Retinal Ganglion Cells.Front Neurosci. 2019 Sep 13;13:979. doi: 10.3389/fnins.2019.00979. eCollection 2019. Front Neurosci. 2019. PMID: 31572123 Free PMC article.
References
-
- Land M. (1992) Predictable eye-head coordination during driving. Nature 359, 318–320. - PubMed
-
- Martinez-Conde S., Macknik S., Hubel D. (2004) The role of fixational eye movements in visual perception. Nat. Rev. Neurosci. 5, 229–240. - PubMed
-
- Lee D., Malpeli J. (1998) Effects of saccades on the activity of neurons in the cat lateral geniculate nucleus. J. Neurophysiol. 79, 922–936. - PubMed
-
- Reppas J., Usrey W., Reid R. (2002) Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron 35, 961–974. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

