Polymeric Materials for Gene Delivery and DNA Vaccination
- PMID: 28413262
- PMCID: PMC5391878
- DOI: 10.1002/adma.200801478
Polymeric Materials for Gene Delivery and DNA Vaccination
Abstract
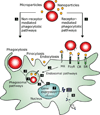
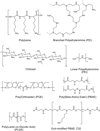
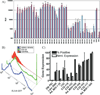
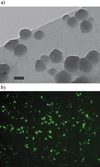
Gene delivery holds great potential for the treatment of many different diseases. Vaccination with DNA holds particular promise, and may provide a solution to many technical challenges that hinder traditional vaccine systems including rapid development and production and induction of robust cell-mediated immune responses. However, few candidate DNA vaccines have progressed past preclinical development and none have been approved for human use. This Review focuses on the recent progress and challenges facing materials design for nonviral DNA vaccine drug delivery systems. In particular, we highlight work on new polymeric materials and their effects on protective immune activation, gene delivery, and current efforts to optimize polymeric delivery systems for DNA vaccination.
Figures








References
-
- List of Vaccine-Preventable Diseases, Centers for Disease Control and Prevention (CDC) [accessed 19 November, 2008]; http://www.cdc.gov/vaccines/vpd-vac/vpd-list.htm.
-
- Robinson HL. Clin. Pharmacol. Ther. 2007;82:686. - PubMed
-
- Berzofsky JA, Ahlers JD, Belyakov IM. Nat. Rev. Immunol. 2001;1:209. - PubMed
-
- Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Annu. Rev. Immunol. 2002;20:621. - PubMed
-
- Sharma AK, Khuller GK. Immunol. Cell Biol. 2001;79:537. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

