Antitumor effect of sunitinib in human prostate cancer cells functions via autophagy
- PMID: 28413468
- PMCID: PMC5377283
- DOI: 10.3892/etm.2017.4134
Antitumor effect of sunitinib in human prostate cancer cells functions via autophagy
Abstract
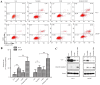
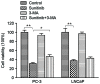
The aim of the present study was to explore sunitinib-induced autophagic effects and the specific molecular mechanisms involved, in vitro, using PC-3 and LNCaP human prostate cancer cell lines. Cells were exposed to escalating doses of sunitinib treatment and subsequent cell viability and cell cycle analyses were performed to evaluate the inhibitory effect of sunitinib in vitro. Immunofluorescence staining of microtubule associated protein 1A/1B-light chain 3 (LC3) puncta was employed to assess autophagy levels after sunitinib treatment. Western blot analysis was performed to evaluate variations in the levels of LC3, sequestosome-1, extracellular signal regulated kinase 1/2 (ERK1/2), mammalian target of rapamycin (mTOR), p70 ribosomal protein S6 kinase (p70S6K) and cleaved caspase-3 proteins. The present study revealed that sunitinib treatment inhibited cell growth and triggered autophagy in a dose-dependent manner in both cell lines. In addition, sunitinib activated ERK1/2 and inhibited mTOR/p70S6K signaling. Sunitinib-induced autophagy was notably reversed by ERK1/2 kinase inhibitor, U0126. Furthermore, inhibition of sunitinib-induced autophagy by 3-methyladenine enhanced apoptosis and exhibited improved cell viability, which indicated that sunitinib induces not only apoptosis but also autophagic cell death in prostate cancer cell lines. These results may lead to an improved understanding of the mechanism of sunitinib's cytotoxic action and may provide evidence that combined sunitinib autophagy-regulating treatment may be of benefit to anti-prostate cancer therapy.
Keywords: apoptosis; autophagy; extracellular signal-regulated kinases 1/2; mechanistic target of rapamycin; prostate cancer cells; sunitinib.
Figures






Similar articles
-
Tanshinone IIA induces autophagic cell death via activation of AMPK and ERK and inhibition of mTOR and p70 S6K in KBM-5 leukemia cells.Phytother Res. 2014 Mar;28(3):458-64. doi: 10.1002/ptr.5015. Epub 2013 Jun 27. Phytother Res. 2014. PMID: 23813779
-
[Sunitinib induces autophagy via suppressing Akt/mTOR pathway in renal cell carcinoma].Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Aug 18;48(4):584-589. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 29263493 Chinese.
-
The pan-inhibitor of Aurora kinases danusertib induces apoptosis and autophagy and suppresses epithelial-to-mesenchymal transition in human breast cancer cells.Drug Des Devel Ther. 2015 Feb 17;9:1027-62. doi: 10.2147/DDDT.S74412. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25733818 Free PMC article.
-
Autophagy Induction by α-Santalol in Human Prostate Cancer Cells.Anticancer Res. 2021 Mar;41(3):1197-1202. doi: 10.21873/anticanres.14876. Anticancer Res. 2021. PMID: 33788710
-
Protective role of autophagy in matrine‑induced gastric cancer cell death.Int J Oncol. 2013 Apr;42(4):1417-26. doi: 10.3892/ijo.2013.1817. Epub 2013 Feb 8. Int J Oncol. 2013. PMID: 23404079
Cited by
-
Unveiling the Molecular Landscape of FOXA1 Mutant Prostate Cancer: Insights and Prospects for Targeted Therapeutic Strategies.Int J Mol Sci. 2023 Oct 31;24(21):15823. doi: 10.3390/ijms242115823. Int J Mol Sci. 2023. PMID: 37958805 Free PMC article.
-
Molecular Mechanisms Underlying Autophagy-Mediated Treatment Resistance in Cancer.Cancers (Basel). 2019 Nov 11;11(11):1775. doi: 10.3390/cancers11111775. Cancers (Basel). 2019. PMID: 31717997 Free PMC article. Review.
-
Guanabenz Sensitizes Glioblastoma Cells to Sunitinib by Inhibiting GADD34-Mediated Autophagic Signaling.Neurotherapeutics. 2021 Apr;18(2):1371-1392. doi: 10.1007/s13311-020-00961-z. Epub 2021 Jan 6. Neurotherapeutics. 2021. PMID: 33410111 Free PMC article.
-
Autophagy, apoptosis, and mitochondria: molecular integration and physiological relevance in skeletal muscle.Am J Physiol Cell Physiol. 2019 Jul 1;317(1):C111-C130. doi: 10.1152/ajpcell.00261.2018. Epub 2019 Apr 24. Am J Physiol Cell Physiol. 2019. PMID: 31017800 Free PMC article.
-
Dual role of autophagy in bone metastasis: mechanistic insights and therapeutic targeting.Am J Clin Exp Urol. 2025 Apr 25;13(2):92-117. doi: 10.62347/QCPV6064. eCollection 2025. Am J Clin Exp Urol. 2025. PMID: 40400996 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
