Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development
- PMID: 28413692
- PMCID: PMC5388068
- DOI: 10.1021/acscatal.7b00094
Photoredox-Mediated Routes to Radicals: The Value of Catalytic Radical Generation in Synthetic Methods Development
Abstract
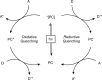
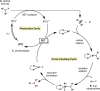
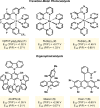
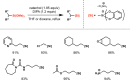
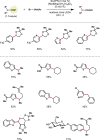
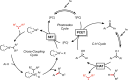
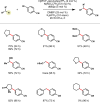
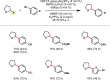
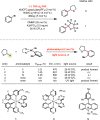
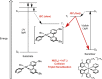
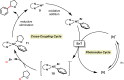
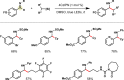
Photoredox catalysis has experienced a revitalized interest from the synthesis community during the past decade. For example, photoredox/Ni dual catalysis protocols have been developed to overcome several inherent limitations of palladium-catalyzed cross-couplings by invoking a single-electron transmetalation pathway. This Perspective highlights advances made by our laboratory since the inception of the photoredox/Ni cross-coupling of benzyltrifluoroborates with aryl bromides. In addition to broadening the scope of trifluoroborate coupling partners, research using readily oxidized hypervalent silicates as radical precursors that demonstrate functional group compatibility is highlighted. The pursuit of electrophilic coupling partners beyond (hetero)aryl bromides has also led to the incorporation of several new classes of C(sp2)-hybridized substrates into light-mediated cross-coupling. Advances to expand the radical toolbox by utilizing feedstock chemicals (e.g., aldehydes) to access radicals that were previously inaccessible from trifluoroborates and silicates are also emphasized. Additionally, several organic photocatalysts have been investigated as replacements for their expensive iridium- and ruthenium-based counterparts. Lastly, the net C-H functionalization of the radical partner in an effort to improve atom economy is presented. An underlying theme in all of these studies is the value of generating radicals in a catalytic manner, rather than stoichiometrically.
Keywords: catalysis; cross-coupling; dual catalysis; nickel; photoredox; radicals.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tellis J. C.; Kelly C. B.; Primer D. N.; Jouffroy M.; Patel N.; Molander G. A. Acc. Chem. Res. 2016, 49, 1429–1439. 10.1021/acs.accounts.6b00214. - DOI - PMC - PubMed
- Prier C. K.; Rankic D. A.; MacMillan D. W. C. Chem. Rev. 2013, 113, 5322–5363. 10.1021/cr300503r. - DOI - PMC - PubMed
- Tucker J. W.; Stephenson C. R. J. J. Org. Chem. 2012, 77, 1617–1622. 10.1021/jo202538x. - DOI - PubMed
- Skubi K. L.; Blum T. R.; Yoon T. P. Chem. Rev. 2016, 116, 10035–10074. 10.1021/acs.chemrev.6b00018. - DOI - PMC - PubMed
-
- Cano-Yelo H.; Deronzier A. Tetrahedron Lett. 1984, 25, 5517–5520. 10.1016/S0040-4039(01)81614-2. - DOI
-
- Ischay M. A.; Anzovino M. E.; Du J.; Yoon T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. 10.1021/ja805387f. - DOI - PubMed
- Du J.; Yoon T. P. J. Am. Chem. Soc. 2009, 131, 14604–14605. 10.1021/ja903732v. - DOI - PMC - PubMed
- Ischay M. A.; Lu Z.; Yoon T. P. J. Am. Chem. Soc. 2010, 132, 8572–8574. 10.1021/ja103934y. - DOI - PMC - PubMed
- Yoon T. P.; Ischay M. A.; Du J. Nat. Chem. 2010, 2, 527–532. 10.1038/nchem.687. - DOI - PubMed
- Hurtley A. E.; Cismesia M. A.; Ischay M. A.; Yoon T. P. Tetrahedron 2011, 67, 4442–4448. 10.1016/j.tet.2011.02.066. - DOI - PMC - PubMed
- Lin S.; Ischay M. A.; Fry C. G.; Yoon T. P. J. Am. Chem. Soc. 2011, 133, 19350–19353. 10.1021/ja2093579. - DOI - PMC - PubMed
- Du J.; Ruiz Espelt L.; Guzei I. A.; Yoon T. P. Chem. Sci. 2011, 2, 2115–2119. 10.1039/c1sc00357g. - DOI - PMC - PubMed
- Lu Z.; Shen M.; Yoon T. P. J. Am. Chem. Soc. 2011, 133, 1162–1164. 10.1021/ja107849y. - DOI - PMC - PubMed
-
- Nagib D. A.; Scott M. E.; MacMillan D. W. C. J. Am. Chem. Soc. 2009, 131, 10875–10877. 10.1021/ja9053338. - DOI - PMC - PubMed
- Shih H. – W.; Vander Wal N.; Grange R. L.; MacMillan D. W. C. J. Am. Chem. Soc. 2010, 132, 13600–13603. 10.1021/ja106593m. - DOI - PMC - PubMed
- Pham P. V.; Nagib D. A.; MacMillan D. W. C. Angew. Chem., Int. Ed. 2011, 50, 6119–6122. 10.1002/anie.201101861. - DOI - PMC - PubMed
- McNally A.; Prier C. K.; MacMillan D. W. C. Science 2011, 334, 1114–1117. 10.1126/science.1213920. - DOI - PMC - PubMed
- Nagib D. A.; MacMillan D. W. C. Nature 2011, 480, 224–228. 10.1038/nature10647. - DOI - PMC - PubMed
-
- Narayanam J. M. R.; Tucker J. W.; Stephenson C. R. J. J. Am. Chem. Soc. 2009, 131, 8756–8757. 10.1021/ja9033582. - DOI - PubMed
- Tucker J. W.; Narayanam J. M. R.; Krabbe S. W.; Stephenson C. R. J. Org. Lett. 2010, 12, 368–371. 10.1021/ol902703k. - DOI - PubMed
- Condie A. G.; Gonzalez-Gomez J. – C.; Stephenson C. R. J. J. Am. Chem. Soc. 2010, 132, 1464–1465. 10.1021/ja909145y. - DOI - PubMed
- Tucker J. W.; Nguyen J. D.; Narayanam J. M. R.; Krabbe S. W.; Stephenson C. R. J. Chem. Commun. 2010, 46, 4985–4987. 10.1039/c0cc00981d. - DOI - PubMed
- Furst L.; Matsuura B. S.; Narayanam J. M. R.; Tucker J. W.; Stephenson C. R. J. Org. Lett. 2010, 12, 3104–3107. 10.1021/ol101146f. - DOI - PubMed
- Narayanam J. M. R.; Stephenson C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. 10.1039/B913880N. - DOI - PubMed
- Dai C.; Narayanam J. M. R.; Stephenson C. R. J. Nat. Chem. 2011, 3, 140–145. 10.1038/nchem.949. - DOI - PubMed
- Nguyen J. D.; Tucker J. W.; Konieczynska M. D.; Stephenson C. R. J. J. Am. Chem. Soc. 2011, 133, 4160–4163. 10.1021/ja108560e. - DOI - PMC - PubMed
- Tucker J. W.; Narayanam J. M. R.; Shah P. S.; Stephenson C. R. J. Chem. Commun. 2011, 47, 5040–5042. 10.1039/c1cc10827a. - DOI - PubMed
- Furst L.; Narayanam J. M. R.; Stephenson C. R. J. Angew. Chem., Int. Ed. 2011, 50, 9655–9659. 10.1002/anie.201103145. - DOI - PMC - PubMed
- Tucker J. W.; Stephenson C. R. J. Org. Lett. 2011, 13, 5468–5471. 10.1021/ol202178t. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
