Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy
- PMID: 28414061
- PMCID: PMC5632581
- DOI: 10.1016/j.jaci.2017.03.028
Mechanistic correlates of clinical responses to omalizumab in the setting of oral immunotherapy for milk allergy
Abstract
Background: In our recent clinical trial, the addition of omalizumab to oral immunotherapy (OIT) for milk allergy improved safety, but no significant clinical benefit was detected.
Objective: We sought to investigate mechanisms by which omalizumab modulates immunity in the context of OIT and to identify baseline biomarkers that predict subgroups of patients most likely to benefit from omalizumab.
Methods: Blood was obtained at baseline and multiple time points during a placebo-controlled trial of OIT for milk allergy in which subjects were randomized to receive omalizumab or placebo. Immunologic outcomes included measurement of basophil CD63 expression and histamine release and casein-specific CD4+ regulatory T-cell proliferation. Biomarkers were analyzed in relationship to measurements of safety and efficacy.
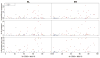
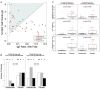
Results: Milk-induced basophil CD63 expression was transiently reduced in whole blood samples from both omalizumab- and placebo-treated subjects. However, IgE-dependent histamine release increased in washed cell preparations from omalizumab- but not placebo-treated subjects. No increase in regulatory T-cell frequency was evident in either group. Subjects with lower rates of adverse reactions, regardless of arm, experienced better clinical outcomes. Pre-OIT basophil reactivity positively associated with occurrence of symptoms during OIT, whereas the baseline milk IgE/total IgE ratio correlated with the likelihood of achieving sustained unresponsiveness. A combination of baseline basophil and serologic biomarkers defined a subset of patients in which adjunctive therapy with omalizumab was associated with attainment of sustained unresponsiveness and a reduction in adverse reactions.
Conclusions: Combining omalizumab therapy with milk OIT led to distinct alterations in basophil reactivity but not T-cell responses. Baseline biomarkers can identify subjects most likely to benefit from adjunctive therapy with omalizumab.
Keywords: Oral immunotherapy; basophil activation; biomarker; desensitization; food allergy; milk allergy; omalizumab; regulatory T cells; sustained unresponsiveness.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Figures





References
-
- Sampson HA. Food-induced anaphylaxis. Novartis Found Symp. 2004;257:161–71. discussion 71-6, 207-10, 76-85. - PubMed
-
- Feuille E, Nowak-Wegrzyn A. Oral Immunotherapy for Food Allergies. Ann Nutr Metab. 2016;68(1):19–31. - PubMed
-
- Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137:973–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

