PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells
- PMID: 28414296
- PMCID: PMC5409099
- DOI: 10.1172/JCI91138
PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells
Abstract
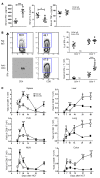
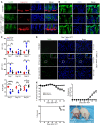
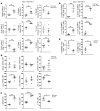
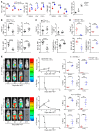
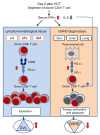
Programmed death ligand-1 (PD-L1) interacts with programmed death-1 (PD-1) and the immunostimulatory molecule CD80 and functions as a checkpoint to regulate immune responses. The interaction of PD-L1 with CD80 alone has been shown to exacerbate the severity of graft-versus-host disease (GVHD), whereas costimulation of CD80 and PD-1 ameliorates GVHD. Here we have demonstrated that temporary depletion of donor CD4+ T cells early after hematopoietic cell transplantation effectively prevents GVHD while preserving strong graft-versus-leukemia (GVL) effects in allogeneic and xenogeneic murine GVHD models. Depletion of donor CD4+ T cells increased serum IFN-γ but reduced IL-2 concentrations, leading to upregulation of PD-L1 expression by recipient tissues and donor CD8+ T cells. In GVHD target tissues, the interactions of PD-L1 with PD-1 on donor CD8+ T cells cause anergy, exhaustion, and apoptosis, thereby preventing GVHD. In lymphoid tissues, the interactions of PD-L1 with CD80 augment CD8+ T cell expansion without increasing anergy, exhaustion, or apoptosis, resulting in strong GVL effects. These results indicate that the outcome of PD-L1-mediated signaling in CD8+ T cells depends on the presence or absence of CD4+ T cells, the nature of the interacting receptor expressed by CD8+ T cells, and the tissue environment in which the signaling occurs.
Conflict of interest statement
Figures




Comment in
- PD-L1 serves as a double agent in separating GVL from GVHD
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

