Transcriptional regulatory logic of the diurnal cycle in the mouse liver
- PMID: 28414715
- PMCID: PMC5393560
- DOI: 10.1371/journal.pbio.2001069
Transcriptional regulatory logic of the diurnal cycle in the mouse liver
Abstract
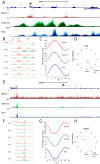
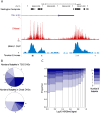
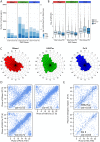
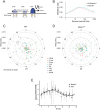
Many organisms exhibit temporal rhythms in gene expression that propel diurnal cycles in physiology. In the liver of mammals, these rhythms are controlled by transcription-translation feedback loops of the core circadian clock and by feeding-fasting cycles. To better understand the regulatory interplay between the circadian clock and feeding rhythms, we mapped DNase I hypersensitive sites (DHSs) in the mouse liver during a diurnal cycle. The intensity of DNase I cleavages cycled at a substantial fraction of all DHSs, suggesting that DHSs harbor regulatory elements that control rhythmic transcription. Using chromatin immunoprecipitation followed by DNA sequencing (ChIP-seq), we found that hypersensitivity cycled in phase with RNA polymerase II (Pol II) loading and H3K27ac histone marks. We then combined the DHSs with temporal Pol II profiles in wild-type (WT) and Bmal1-/- livers to computationally identify transcription factors through which the core clock and feeding-fasting cycles control diurnal rhythms in transcription. While a similar number of mRNAs accumulated rhythmically in Bmal1-/- compared to WT livers, the amplitudes in Bmal1-/- were generally lower. The residual rhythms in Bmal1-/- reflected transcriptional regulators mediating feeding-fasting responses as well as responses to rhythmic systemic signals. Finally, the analysis of DNase I cuts at nucleotide resolution showed dynamically changing footprints consistent with dynamic binding of CLOCK:BMAL1 complexes. Structural modeling suggested that these footprints are driven by a transient heterotetramer binding configuration at peak activity. Together, our temporal DNase I mappings allowed us to decipher the global regulation of diurnal transcription rhythms in the mouse liver.
Conflict of interest statement
BW and FG are employess of Nestlé Institute of Health Sciences SA.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

