Models of human adamantinomatous craniopharyngioma tissue: Steps toward an effective adjuvant treatment
- PMID: 28414888
- PMCID: PMC8028941
- DOI: 10.1111/bpa.12499
Models of human adamantinomatous craniopharyngioma tissue: Steps toward an effective adjuvant treatment
Abstract
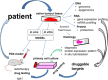
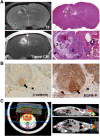
Even though ACP is a benign tumor, treatment is challenging because of the tumor's eloquent location. Today, with the exception of surgical intervention and irradiation, further treatment options are limited. However, ongoing molecular research in this field provides insights into the pathways involved in ACP pathogenesis and reveal a plethora of druggable targets. In the next step, appropriate models are essential to identify the most suitable and effective substances for clinical practice. Primary cell cultures in low passages provide a proper and rapid tool for initial drug potency testing. The patient-derived xenograft (PDX) model accommodates ACP complexity in that it shows respect to the preserved architecture and similar histological appearance to human tumors and therefore provides the most appropriate means for analyzing pharmacological efficacy. Nevertheless, further research is needed to understand in more detail the biological background of ACP pathogenesis, which provides the identification of the best targets in the hierarchy of signaling cascades. ACP models are also important for the continuous testing of new targeting drugs, to establish precision medicine.
Keywords: EGFR; PDX model; craniopharyngioma; primary cell cultures; radioresistance.
© 2017 International Society of Neuropathology.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Drug priming enhances radiosensitivity of adamantinomatous craniopharyngioma via downregulation of survivin.Neurosurg Focus. 2016 Dec;41(6):E14. doi: 10.3171/2016.9.FOCUS16316. Neurosurg Focus. 2016. PMID: 27903123
-
Molecular pathology of adamantinomatous craniopharyngioma: review and opportunities for practice.Neurosurg Focus. 2016 Dec;41(6):E4. doi: 10.3171/2016.8.FOCUS16307. Neurosurg Focus. 2016. PMID: 27903120 Review.
-
Osteoblastic differentiation and cell calcification of adamantinomatous craniopharyngioma induced by bone morphogenetic protein-2.Cancer Biomark. 2017;18(2):191-198. doi: 10.3233/CBM-161576. Cancer Biomark. 2017. PMID: 27983534
-
Effects of Simvastatin on Inflammatory Response and Biological Behaviour of Adamantinomatous Craniopharyngioma.Neuroendocrinology. 2024;114(10):934-949. doi: 10.1159/000539821. Epub 2024 Jul 22. Neuroendocrinology. 2024. PMID: 38964285
-
First evidence of anti-VEGF efficacy in an adult case of adamantinomatous craniopharyngioma: Case report and illustrative review.Ann Endocrinol (Paris). 2023 Dec;84(6):727-733. doi: 10.1016/j.ando.2023.10.003. Epub 2023 Oct 19. Ann Endocrinol (Paris). 2023. PMID: 37865272 Review.
Cited by
-
Investigating the Protein Signature of Adamantinomatous Craniopharyngioma Pediatric Brain Tumor Tissue: Towards the Comprehension of Its Aggressive Behavior.Dis Markers. 2019 May 2;2019:3609789. doi: 10.1155/2019/3609789. eCollection 2019. Dis Markers. 2019. PMID: 31191748 Free PMC article.
-
Feasibility of primary human cell cultures as a model for adamantinomatous craniopharyngioma research: Evidence from RNA-Seq analysis.Oncol Lett. 2020 Mar;19(3):2346-2354. doi: 10.3892/ol.2020.11309. Epub 2020 Jan 16. Oncol Lett. 2020. PMID: 32194734 Free PMC article.
-
Characterization of the murine orthotopic adamantinomatous craniopharyngioma PDX model by MRI in correlation with histology.PLoS One. 2018 May 24;13(5):e0197895. doi: 10.1371/journal.pone.0197895. eCollection 2018. PLoS One. 2018. PMID: 29795641 Free PMC article.
References
-
- Buslei R, Holsken A, Hofmann B, Kreutzer J, Siebzehnrubl F, Hans V et al (2007) Nuclear beta‐ catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol 113:585–590. - PubMed
-
- Buslei R, Nolde M, Hofmann B, Meissner S, Eyupoglu IY, Siebzehnrubl F et al (2005) Common mutations of beta‐catenin in adamantinomatous craniopharyngiomas but not in other tumors originating from the sellar region. Acta Neuropathol (Berl) 109:589–597. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

