Selective HDAC inhibitors with potent oral activity against leukemia and colorectal cancer: Design, structure-activity relationship and anti-tumor activity study
- PMID: 28415009
- PMCID: PMC5994920
- DOI: 10.1016/j.ejmech.2017.03.069
Selective HDAC inhibitors with potent oral activity against leukemia and colorectal cancer: Design, structure-activity relationship and anti-tumor activity study
Abstract
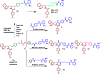
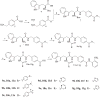
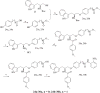
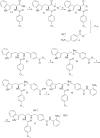
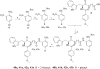
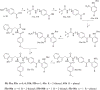
Previously, we reported the discovery of a series of N-hydroxycinnamamide-based HDAC inhibitors, among which compound 11y exhibited high HDAC1/3 selectivity. In this current study, structural derivatization of 11y led to a new series of benzamide based HDAC inhibitors. Most of the compounds exhibited high HDACs inhibitory potency. Compound 11a (with 4-methoxybenzoyl as N-substituent in the cap and 4-(aminomethyl) benzoyl as the linker group) exhibited selectivity against HDAC1 to some extent, and showed potent antiproliferative activity against several tumor cell lines. In vivo studies revealed that compound 11a displayed potent oral antitumor activity in both hematological tumor cell U937 xenograft model and solid tumor cell HCT116 xenograft model with no obvious toxicity. Further modification of benzamide 3, 11a and 19 afforded new thienyl and phenyl compounds (50a, 50b, 63a, 63b and 63c) with dramatic HDAC1 and HDAC2 dual selectivity, and the fluorine containing compound 56, with moderate HDAC3 selectivity.
Keywords: Anti-tumor drugs; Benzamides; HDACIs; HDACs; In vivo oral activity.
Copyright © 2017 Elsevier Masson SAS. All rights reserved.
Figures




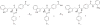
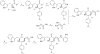
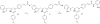
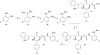
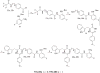
Similar articles
-
Discovery of quinazolinyl-containing benzamides derivatives as novel HDAC1 inhibitors with in vitro and in vivo antitumor activities.Bioorg Chem. 2021 Dec;117:105407. doi: 10.1016/j.bioorg.2021.105407. Epub 2021 Oct 6. Bioorg Chem. 2021. PMID: 34653945
-
Synthesis, biological evaluation, and molecular docking analysis of novel linker-less benzamide based potent and selective HDAC3 inhibitors.Bioorg Chem. 2021 Sep;114:105050. doi: 10.1016/j.bioorg.2021.105050. Epub 2021 Jun 4. Bioorg Chem. 2021. PMID: 34120025
-
Development of 3-hydroxycinnamamide-based HDAC inhibitors with potent in vitro and in vivo anti-tumor activity.Eur J Med Chem. 2015 Jan 7;89:628-37. doi: 10.1016/j.ejmech.2014.10.077. Epub 2014 Oct 29. Eur J Med Chem. 2015. PMID: 25462271
-
HDAC3 is a potential validated target for cancer: An overview on the benzamide-based selective HDAC3 inhibitors through comparative SAR/QSAR/QAAR approaches.Eur J Med Chem. 2018 Sep 5;157:1127-1142. doi: 10.1016/j.ejmech.2018.08.081. Epub 2018 Aug 29. Eur J Med Chem. 2018. PMID: 30179749 Review.
-
HDAC1/3 dual selective inhibitors - new therapeutic agents for the potential treatment of cancer.Drug Discov Ther. 2014 Oct;8(5):225-8. doi: 10.5582/ddt.2014.01034. Drug Discov Ther. 2014. PMID: 25382558 Review.
Cited by
-
Non-Hydroxamate Zinc-Binding Groups as Warheads for Histone Deacetylases.Molecules. 2021 Aug 25;26(17):5151. doi: 10.3390/molecules26175151. Molecules. 2021. PMID: 34500583 Free PMC article. Review.
-
Design and Synthesis of Hybrid Compounds as Epigenetic Modifiers.Pharmaceuticals (Basel). 2021 Dec 15;14(12):1308. doi: 10.3390/ph14121308. Pharmaceuticals (Basel). 2021. PMID: 34959707 Free PMC article.
-
Development and Biological Evaluation of the First Highly Potent and Specific Benzamide-Based Radiotracer [18F]BA3 for Imaging of Histone Deacetylases 1 and 2 in Brain.Pharmaceuticals (Basel). 2022 Mar 8;15(3):324. doi: 10.3390/ph15030324. Pharmaceuticals (Basel). 2022. PMID: 35337122 Free PMC article.
-
Targeting histone deacetylases for cancer therapy: Trends and challenges.Acta Pharm Sin B. 2023 Jun;13(6):2425-2463. doi: 10.1016/j.apsb.2023.02.007. Epub 2023 Feb 18. Acta Pharm Sin B. 2023. PMID: 37425042 Free PMC article. Review.
-
Can social adversity alter the epigenome, trigger oral disease, and affect future generations?Ir J Med Sci. 2024 Oct;193(5):2597-2606. doi: 10.1007/s11845-024-03697-3. Epub 2024 May 14. Ir J Med Sci. 2024. PMID: 38740675 Free PMC article. Review.
References
-
- Kristensen LS, Nielsen HM, Hansen LL. Epigenetics and cancer treatment. Eur J Pharmacol. 2009;625:131–142. - PubMed
-
- Roche J, Bertrand P. Inside HDACs with more selective HDAC inhibitors. Eur J Med Chem. 2016;121:451–483. - PubMed
-
- Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications[J] Mol Cancer Ther. 2009;8:1409–1420. - PubMed
-
- Giannini G, Cabri W, Fattorusso C, Rodriquez M. Histone deacetylase inhibitors in the treatment of cancer: overview and perspectives. Future Med Chem. 2012;4:1439–1460. - PubMed
-
- Kumar A, Chauhan S. How much successful are the medicinal chemists in modulation of SIRT1: a critical review. Eur J Med Chem. 2016;119:45–69. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

