Endocytosis of tight junction proteins and the regulation of degradation and recycling
- PMID: 28415156
- PMCID: PMC5479724
- DOI: 10.1111/nyas.13346
Endocytosis of tight junction proteins and the regulation of degradation and recycling
Abstract
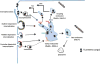
Internalization of tight junction (TJ) proteins from the plasma membrane is a pivotal mechanism regulating TJ plasticity and function in both epithelial and endothelial barrier tissues. Once internalized, the TJ proteins enter complex vesicular machinery, where further trafficking is directly dependent on the initiating stimulus and downstream signaling pathways that regulate the sorting and destiny of TJ proteins, as well as on cell and barrier responses. The destiny of internalized TJ proteins is recycling to the plasma membrane or sorting to late endosomes and degradation. This review highlights recent advances in our knowledge of endocytosis and vesicular trafficking of TJ proteins in both epithelial and endothelial cells. A greater understanding of these processes may allow for the development of methods to modulate barrier permeability for drug delivery or prevent barrier dysfunction in disease states.
Keywords: claudin; degradation; early endosomes; late endosomes; recycling.
© 2017 New York Academy of Sciences.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiological reviews. 2004;84:869–901. - PubMed
-
- Haseloff RF, Dithmer S, Winkler L, et al. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Seminars in cell & developmental biology. 2015;38:16–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

