Interleukin-2-regulatory T cell axis critically regulates maintenance of hematopoietic stem cells
- PMID: 28415569
- PMCID: PMC5444691
- DOI: 10.18632/oncotarget.16377
Interleukin-2-regulatory T cell axis critically regulates maintenance of hematopoietic stem cells
Abstract
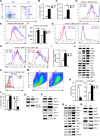
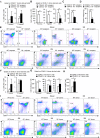
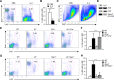
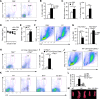
The role of IL-2 in HSC maintenance is unknown. Here we show that Il2-/- mice develop severe anomalies in HSC maintenance leading to defective hematopoiesis. Whereas, lack of IL-2 signaling was detrimental for lympho- and erythropoiesis, myelopoiesis was enhanced in Il2-/- mice. Investigation of the underlying mechanisms of dysregulated hematopoiesis in Il2-/- mice shows that the IL-2-Treg cell axis is indispensable for HSC maintenance and normal hematopoiesis. Lack of Treg activity resulted in increased IFN-γ production by activated T cells and an expansion of the HSCs in the bone marrow (BM). Though, restoring Treg population successfully rescued HSC maintenance in Il2-/- mice, preventing IFN-γ activity could do the same even in the absence of Treg cells. Our study suggests that equilibrium in IL-2 and IFN-γ activity is critical for steady state hematopoiesis, and in clinical conditions of BM failure, IL-2 or anti-IFN-γ treatment might help to restore hematopoiesis.
Keywords: IFN-γ; IL-10; IL-2; Immune response; Immunity; Immunology and Microbiology Section; Treg cells; hematopoietic stem cells.
Conflict of interest statement
The authors declare that they have no financial or commercial conflict of interest.
Figures

Similar articles
-
Interleukin-2 critically regulates bone marrow erythropoiesis and prevents anemia development.Eur J Immunol. 2015 Dec;45(12):3362-74. doi: 10.1002/eji.201545596. Epub 2015 Oct 15. Eur J Immunol. 2015. PMID: 26404745
-
Hematopoietic stem cell functional failure in interleukin-2-deficient mice.J Hematother Stem Cell Res. 2002 Dec;11(6):905-12. doi: 10.1089/152581602321080565. J Hematother Stem Cell Res. 2002. PMID: 12590705
-
IL-2 activation of STAT5 enhances production of IL-10 from human cytotoxic regulatory T cells, HOZOT.Exp Hematol. 2008 Feb;36(2):181-92. doi: 10.1016/j.exphem.2007.09.010. Epub 2007 Nov 26. Exp Hematol. 2008. PMID: 18023521
-
The Regulatory Role of IFN-γ on the Proliferation and Differentiation of Hematopoietic Stem and Progenitor Cells.Stem Cell Rev Rep. 2017 Dec;13(6):705-712. doi: 10.1007/s12015-017-9761-1. Stem Cell Rev Rep. 2017. PMID: 28852997 Review.
-
Impact of interferon-γ on hematopoiesis.Blood. 2014 Oct 16;124(16):2479-86. doi: 10.1182/blood-2014-04-568451. Epub 2014 Sep 3. Blood. 2014. PMID: 25185711 Review.
Cited by
-
Sarcodon imbricatus polysaccharides improve mouse hematopoietic function after cyclophosphamide-induced damage via G-CSF mediated JAK2/STAT3 pathway.Cell Death Dis. 2018 May 21;9(6):578. doi: 10.1038/s41419-018-0634-6. Cell Death Dis. 2018. PMID: 29784961 Free PMC article.
-
NFAT-mediated defects in erythropoiesis cause anemia in Il2-/- mice.Oncotarget. 2017 Dec 28;9(11):9632-9644. doi: 10.18632/oncotarget.23745. eCollection 2018 Feb 9. Oncotarget. 2017. PMID: 29515759 Free PMC article.
-
Prolonged haematologic toxicity in CAR-T-cell therapy: A review.J Cell Mol Med. 2023 Dec;27(23):3662-3671. doi: 10.1111/jcmm.17930. Epub 2023 Sep 13. J Cell Mol Med. 2023. PMID: 37702530 Free PMC article. Review.
-
Aging alters the epigenetic asymmetry of HSC division.PLoS Biol. 2018 Sep 20;16(9):e2003389. doi: 10.1371/journal.pbio.2003389. eCollection 2018 Sep. PLoS Biol. 2018. PMID: 30235201 Free PMC article.
-
Anoxia Rapidly Induces Changes in Expression of a Large and Diverse Set of Genes in Endothelial Cells.Int J Mol Sci. 2023 Mar 8;24(6):5157. doi: 10.3390/ijms24065157. Int J Mol Sci. 2023. PMID: 36982232 Free PMC article.
References
-
- Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

