GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression
- PMID: 28415589
- PMCID: PMC5482628
- DOI: 10.18632/oncotarget.16411
GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression
Abstract
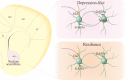
Background: Major depression, persistent low mood, is one of common psychiatric diseases. Chronic stressful life is believed to be a major risk factor that leads to dysfunctions of the limbic system. However, a large number of the individuals with experiencing chronic stress do not suffer from major depression, called as resilience. Endogenous mechanisms underlying neuronal invulnerability to chronic stress versus major depression are largely unknown. As GABAergic neurons are vulnerable to chronic stress and their impairments is associated with major depression, we have examined whether the invulnerability of GABAergic neurons in the limbic system is involved in resilience.
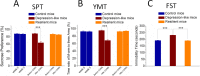
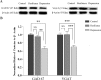
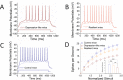
Results: GABAergic neurons in the nucleus accumbens from depression-like mice induced by chronic unpredictable mild stress appear the decreases in their GABA release, spiking capability and excitatory input reception, compared with those in resilience mice. The levels of decarboxylase and vesicular GABA transporters decrease in depression-like mice, but not resilience.
Materials and methods: Mice were treated by chronic unpredictable mild stress for three weeks. Depression-like behaviors or resilience was confirmed by seeing whether their behaviors change significantly in sucrose preference, Y-maze and forced swimming tests. Mice from controls as well as depression and resilience in response to chronic unpredictable mild stress were studied in terms of GABAergic neuron activity in the nucleus accumbens by cell electrophysiology and protein chemistry.
Conclusions: The impairment of GABAergic neurons in the nucleus accumbens is associated with major depression. The invulnerability of GABAergic neurons to chronic stress may be one of cellular mechanisms for the resilience to chronic stress.
Keywords: depression; neuron; nucleus accumbens; resilience; synapse.
Conflict of interest statement
All authors declare no competing interest. All authors have read and approved the final version of the manuscript.
Figures


Similar articles
-
Incoordination among Subcellular Compartments Is Associated with Depression-Like Behavior Induced by Chronic Mild Stress.Int J Neuropsychopharmacol. 2016 Apr 29;19(5):pyv122. doi: 10.1093/ijnp/pyv122. Print 2016 May. Int J Neuropsychopharmacol. 2016. PMID: 26506857 Free PMC article.
-
microRNA and mRNA profiles in nucleus accumbens underlying depression versus resilience in response to chronic stress.Am J Med Genet B Neuropsychiatr Genet. 2018 Sep;177(6):563-579. doi: 10.1002/ajmg.b.32651. Epub 2018 Aug 14. Am J Med Genet B Neuropsychiatr Genet. 2018. PMID: 30105773 Free PMC article.
-
microRNA and mRNA profiles in ventral tegmental area relevant to stress-induced depression and resilience.Prog Neuropsychopharmacol Biol Psychiatry. 2018 Aug 30;86:150-165. doi: 10.1016/j.pnpbp.2018.05.023. Epub 2018 Jun 1. Prog Neuropsychopharmacol Biol Psychiatry. 2018. PMID: 29864451
-
[Roles of altered striatal function in major depression].Brain Nerve. 2012 Aug;64(8):919-26. Brain Nerve. 2012. PMID: 22868883 Review. Japanese.
-
Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression.Biol Psychiatry. 2017 Apr 15;81(8):645-653. doi: 10.1016/j.biopsych.2016.09.007. Epub 2016 Sep 15. Biol Psychiatry. 2017. PMID: 27871668 Free PMC article. Review.
Cited by
-
NAc-DBS corrects depression-like behaviors in CUMS mouse model via disinhibition of DA neurons in the VTA.Mol Psychiatry. 2024 May;29(5):1550-1566. doi: 10.1038/s41380-024-02476-x. Epub 2024 Feb 15. Mol Psychiatry. 2024. PMID: 38361128
-
Moderate maternal separation mitigates the altered synaptic transmission and neuronal activation in amygdala by chronic stress in adult mice.Mol Brain. 2019 Dec 18;12(1):111. doi: 10.1186/s13041-019-0534-4. Mol Brain. 2019. PMID: 31849343 Free PMC article.
-
Efficacy and safety of a GABAergic drug (Gamalate® B6): effects on behavior and cognition in young adults with borderline-to-mild intellectual developmental disabilities and ADHD.Drugs Context. 2020 Jan 23;9:212601. doi: 10.7573/dic.212601. eCollection 2020. Drugs Context. 2020. PMID: 32158489 Free PMC article.
-
Piriform cortical glutamatergic and GABAergic neurons express coordinated plasticity for whisker-induced odor recall.Oncotarget. 2017 Sep 23;8(56):95719-95740. doi: 10.18632/oncotarget.21207. eCollection 2017 Nov 10. Oncotarget. 2017. PMID: 29221161 Free PMC article.
-
miR-181b inhibits chemoresistance in cisplatin-resistant H446 small cell lung cancer cells by targeting Bcl-2.Arch Med Sci. 2018 Jun;14(4):745-751. doi: 10.5114/aoms.2018.73131. Epub 2018 Feb 2. Arch Med Sci. 2018. PMID: 30002690 Free PMC article.
References
-
- Klengel T, Binder EB. Gene-environment interactions in major depressive disorder. Can J Psychiatry. 2013;58:76–83. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

