A meta-analysis of randomized controlled trials comparing the efficacy and safety of anastrozole versus tamoxifen for breast cancer
- PMID: 28415634
- PMCID: PMC5564654
- DOI: 10.18632/oncotarget.16466
A meta-analysis of randomized controlled trials comparing the efficacy and safety of anastrozole versus tamoxifen for breast cancer
Abstract
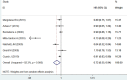
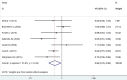
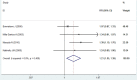
Whether anastrozole has superior effects to tamoxifen for breast cancer remains controversial. Therefore, we conducted this meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of anastrozole versus tamoxifen as adjuvant therapy in breast cancer. A systematic literature search of PubMed, Web of Science, Embase, and Cochrane library were performed to evaluate the survival benefits and toxicity profiles of patients with breast cancer who were treated with anastrozole or tamoxifen. The main outcome measures included disease-free survival (DFS), recurrence-free survival (RFS), overall survival (OS), overall response rate (ORR), and adverse events. Hazard ratios (HRs) or risk ratios (RRs) with 95% confidence intervals (CIs) were pooled using a fixed-effects model or random-effects model. Nine RCTs with a total of 15,300 patients met the inclusion criteria and were included in this meta-analysis. Pooled estimates suggested that, anastrozole was associated with a significantly improvement in DFS (HR=0.72, 95%CI: 0.55-0.94; P=0.016), and ORR (RR=1.21, 95% CI: 1.05-1.39; P=0.009) than tamoxifen. But it did not prolong OS (HR=0.96, 95%CI: 0.77-1.21; P=0.751). Compared with tamoxifen, anastrozole induced a higher incidence of arthralgia (RR=1.55, 95%CI: 1.20-1.99; P=0.001) and bone pain (RR=1.31, 95%CI: 1.05-1.62; P=0.015), as well as a lower incidence of vaginal bleeding (RR=0.51, 95%CI: 0.28-0.93; P=0.029), vaginal discharge (RR=0.31, 95%CI: 0.12-0.82; P=0.017), and thromboembolic events (RR=0.39, 95%CI: 0.28-0.55; P<0.001). Based on the current evidence, patients with breast cancer would benefit from the anastrozole treatment.
Keywords: anastrozole; breast cancer; meta-analysis; tamoxifen.
Conflict of interest statement
All the authors declare that they have no conflict of interest.
Figures


Similar articles
-
Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early-stage breast cancer: results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses.Cancer. 2003 Nov 1;98(9):1802-10. doi: 10.1002/cncr.11745. Cancer. 2003. PMID: 14584060 Clinical Trial.
-
'Arimidex' (anastrozole) versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer--efficacy overview.J Steroid Biochem Mol Biol. 2003 Sep;86(3-5):399-403. doi: 10.1016/s0960-0760(03)00350-9. J Steroid Biochem Mol Biol. 2003. PMID: 14623537 Clinical Trial.
-
Switching to anastrozole versus continued tamoxifen treatment of early breast cancer: preliminary results of the Italian Tamoxifen Anastrozole Trial.J Clin Oncol. 2005 Aug 1;23(22):5138-47. doi: 10.1200/JCO.2005.04.120. Epub 2005 Jul 11. J Clin Oncol. 2005. PMID: 16009955 Clinical Trial.
-
Three years' follow-up from the ATAC trial is sufficient to change clinical practice: a debate.Breast Cancer Res Treat. 2003;80 Suppl 1:S3-11; discussion S13-8. doi: 10.1023/a:1025455130476. Breast Cancer Res Treat. 2003. PMID: 14535530 Review.
-
Reducing the risk for breast cancer recurrence after completion of tamoxifen treatment in postmenopausal women.Clin Ther. 2007 Aug;29(8):1535-47. doi: 10.1016/j.clinthera.2007.08.013. Clin Ther. 2007. PMID: 17919537 Review.
Cited by
-
The Cytochrome CYP4 in Breast and Other Cancers.Biology (Basel). 2025 Jul 4;14(7):812. doi: 10.3390/biology14070812. Biology (Basel). 2025. PMID: 40723371 Free PMC article. Review.
-
Ferrocifen Loaded Lipid Nanocapsules: A Promising Anticancer Medication against Multidrug Resistant Tumors.Cancers (Basel). 2021 May 11;13(10):2291. doi: 10.3390/cancers13102291. Cancers (Basel). 2021. PMID: 34064748 Free PMC article. Review.
-
Tailoring Potential Natural Compounds for the Treatment of Luminal Breast Cancer.Pharmaceuticals (Basel). 2023 Oct 16;16(10):1466. doi: 10.3390/ph16101466. Pharmaceuticals (Basel). 2023. PMID: 37895937 Free PMC article. Review.
-
Data-Driven identification of chemopreventive agents for breast cancer.Turk J Med Sci. 2020 Nov 3;50(SI-2):1691-1696. doi: 10.3906/sag-2003-138. Turk J Med Sci. 2020. PMID: 32233182 Free PMC article. Review.
-
Aromatase inhibitors and risk of cardiovascular events in breast cancer patients: a systematic review and meta-analysis.BMC Pharmacol Toxicol. 2019 Oct 29;20(1):62. doi: 10.1186/s40360-019-0339-1. BMC Pharmacol Toxicol. 2019. PMID: 31665091 Free PMC article.
References
-
- Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, Saunders C, Roche N, Mansel RE, von Minckwitz G, Bonanni B, Palva T, Howell A. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–8. doi: 10.1016/s0140-6736(13)62292-8. - DOI - PubMed
-
- Dodwell D, Clements K, Lawrence G, Kearins O, Thomson CS, Dewar J, Bishop H. Radiotherapy following breast-conserving surgery for screen-detected ductal carcinoma in situ: indications and utilisation in the UK. Interim findings from the Sloane Project. Br J Cancer. 2007;97:725–9. doi: 10.1038/sj.bjc.6603945. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

