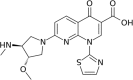
Enhancement of radiosensitivity by the novel anticancer quinolone derivative vosaroxin in preclinical glioblastoma models
- PMID: 28415741
- PMCID: PMC5444710
- DOI: 10.18632/oncotarget.16168
Enhancement of radiosensitivity by the novel anticancer quinolone derivative vosaroxin in preclinical glioblastoma models
Abstract
Purpose: Glioblastoma multiforme (GBM) is the most aggressive brain tumor. The activity of vosaroxin, a first-in-class anticancer quinolone derivative that intercalates DNA and inhibits topoisomerase II, was investigated in GBM preclinical models as a single agent and combined with radiotherapy (RT).
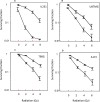
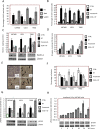
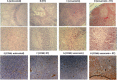
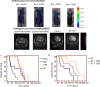
Results: Vosaroxin showed antitumor activity in clonogenic survival assays, with IC50 of 10-100 nM, and demonstrated radiosensitization. Combined treatments exhibited significantly higher γH2Ax levels compared with controls. In xenograft models, vosaroxin reduced tumor growth and showed enhanced activity with RT; vosaroxin/RT combined was more effective than temozolomide/RT. Vosaroxin/RT triggered rapid and massive cell death with characteristics of necrosis. A minor proportion of treated cells underwent caspase-dependent apoptosis, in agreement with in vitro results. Vosaroxin/RT inhibited RT-induced autophagy, increasing necrosis. This was associated with increased recruitment of granulocytes, monocytes, and undifferentiated bone marrow-derived lymphoid cells. Pharmacokinetic analyses revealed adequate blood-brain penetration of vosaroxin. Vosaroxin/RT increased disease-free survival (DFS) and overall survival (OS) significantly compared with RT, vosaroxin alone, temozolomide, and temozolomide/RT in the U251-luciferase orthotopic model.
Materials and methods: Cellular, molecular, and antiproliferative effects of vosaroxin alone or combined with RT were evaluated in 13 GBM cell lines. Tumor growth delay was determined in U87MG, U251, and T98G xenograft mouse models. (DFS) and (OS) were assessed in orthotopic intrabrain models using luciferase-transfected U251 cells by bioluminescence and magnetic resonance imaging.
Conclusions: Vosaroxin demonstrated significant activity in vitro and in vivo in GBM models, and showed additive/synergistic activity when combined with RT in O6-methylguanine methyltransferase-negative and -positive cell lines.
Keywords: double-strand breaks; glioblastoma; radiotherapy; topoisomerase II; vosaroxin.
Conflict of interest statement
JAF was previously employed by and is a consultant for Sunesis Pharmaceuticals. GLG, AM, CM, FV, FM, AC, GR, LV, AV, EDC and CF have no conflicts of interest to disclose.
Figures




References
-
- Polley MY, Lamborn KR, Chang SM, Butowski N, Clarke JL, Prados M. Six-month progression-free survival as an alternative primary efficacy endpoint to overall survival in newly diagnosed glioblastoma patients receiving temozolomide. Neuro Oncol. 2010;12:274–82. doi: 10.1093/neuonc/nop034. - DOI - PMC - PubMed
-
- Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, Meyer B, Simon M, group SNs Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

