hMAGEA2 promotes progression of breast cancer by regulating Akt and Erk1/2 pathways
- PMID: 28415749
- PMCID: PMC5514895
- DOI: 10.18632/oncotarget.16184
hMAGEA2 promotes progression of breast cancer by regulating Akt and Erk1/2 pathways
Abstract
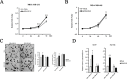
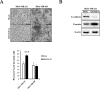
Breast cancer is the most abundant cancer worldwide and a severe problem for women. Notably, breast cancer has a high mortality rate, mainly because of tumor progression and metastasis. Triple-negative breast cancer (TNBC) is highly progressive and lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Therefore, there are no established therapeutic targets against TNBC. In this study, we investigated whether the expression of human melanoma-associated antigen A2 (MAGEA2) is associated with TNBC. We found that hMAGEA2 is significantly overexpressed in human TNBC tissues; we also observed oncogenic properties using TNBC cell lines (MDA-MB-231 and MDA-MB-468). The overexpression of hMAGEA2 in MDA-MB-231 cell line showed dramatically increased cellular proliferation, colony formation, invasion, and xenograft tumor formation and growth. Conversely, knockdown of hMAEGA2 in MDA-MB-468 cell line suppressed cellular proliferation, colony formation, and xenograft tumor formation. Additionally, we showed that hMAGEA2 regulated the activation of Akt and Erk1/2 signaling pathways. These data indicate that hMAGEA2 is important for progression of TNBC and may serve as a novel molecular therapeutic target.
Keywords: Akt; breast cancer; hMAGEA2; metastasis; triple-negative breast cancer.
Conflict of interest statement
The authors declare no potential conflicts of interest
Figures





Similar articles
-
AHNAK suppresses tumour proliferation and invasion by targeting multiple pathways in triple-negative breast cancer.J Exp Clin Cancer Res. 2017 May 12;36(1):65. doi: 10.1186/s13046-017-0522-4. J Exp Clin Cancer Res. 2017. PMID: 28494797 Free PMC article.
-
Calycosin inhibits triple-negative breast cancer progression through down-regulation of the novel estrogen receptor-α splice variant ER-α30-mediated PI3K/AKT signaling pathway.Phytomedicine. 2023 Sep;118:154924. doi: 10.1016/j.phymed.2023.154924. Epub 2023 Jun 14. Phytomedicine. 2023. PMID: 37393829
-
FOXM1 regulates expression of eukaryotic elongation factor 2 kinase and promotes proliferation, invasion and tumorgenesis of human triple negative breast cancer cells.Oncotarget. 2016 Mar 29;7(13):16619-35. doi: 10.18632/oncotarget.7672. Oncotarget. 2016. PMID: 26918606 Free PMC article.
-
Recent treatment progress of triple negative breast cancer.Prog Biophys Mol Biol. 2020 Mar;151:40-53. doi: 10.1016/j.pbiomolbio.2019.11.007. Epub 2019 Nov 21. Prog Biophys Mol Biol. 2020. PMID: 31761352 Review.
-
Glucocorticoid receptors orchestrate a convergence of host and cellular stress signals in triple negative breast cancer.J Steroid Biochem Mol Biol. 2024 Oct;243:106575. doi: 10.1016/j.jsbmb.2024.106575. Epub 2024 Jun 29. J Steroid Biochem Mol Biol. 2024. PMID: 38950871 Review.
Cited by
-
Jazf1 promotes prostate cancer progression by activating JNK/Slug.Oncotarget. 2017 Dec 12;9(1):755-765. doi: 10.18632/oncotarget.23146. eCollection 2018 Jan 2. Oncotarget. 2017. PMID: 29416651 Free PMC article.
-
Transcriptome sequencing to detect the potential role of long non-coding RNAs in bovine mammary gland during the dry and lactation period.BMC Genomics. 2018 Aug 13;19(1):605. doi: 10.1186/s12864-018-4974-5. BMC Genomics. 2018. PMID: 30103699 Free PMC article.
-
Overexpression of melanoma-associated antigen A2 has a clinical significance in embryonal carcinoma and is associated with tumor progression.J Cancer Res Clin Oncol. 2022 Mar;148(3):609-631. doi: 10.1007/s00432-021-03859-1. Epub 2021 Nov 27. J Cancer Res Clin Oncol. 2022. PMID: 34837545 Free PMC article.
-
Prognostic roles of MAGE family members in breast cancer based on KM-Plotter Data.Oncol Lett. 2019 Oct;18(4):3501-3516. doi: 10.3892/ol.2019.10722. Epub 2019 Aug 6. Oncol Lett. 2019. PMID: 31516568 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

