Aspirin regulation of c-myc and cyclinD1 proteins to overcome tamoxifen resistance in estrogen receptor-positive breast cancer cells
- PMID: 28415819
- PMCID: PMC5444740
- DOI: 10.18632/oncotarget.16325
Aspirin regulation of c-myc and cyclinD1 proteins to overcome tamoxifen resistance in estrogen receptor-positive breast cancer cells
Abstract
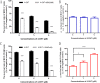
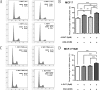
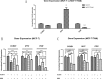
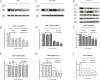
Tamoxifen is still the most commonly used endocrine therapy drug for estrogen receptor (ER)-positive breast cancer patients and has an excellent outcome, but tamoxifen resistance remains a great impediment to successful treatment. Recent studies have prompted an anti-tumor effect of aspirin. Here, we demonstrated that aspirin not only inhibits the growth of ER-positive breast cancer cell line MCF-7, especially when combined with tamoxifen, but also has a potential function to overcome tamoxifen resistance in MCF-7/TAM. Aspirin combined with tamoxifen can down regulate cyclinD1 and block cell cycle in G0/G1 phase. Besides, tamoxifen alone represses c-myc, progesterone receptor (PR) and cyclinD1 in MCF-7 cell line but not in MCF-7/TAM, while aspirin combined with tamoxifen can inhibit the expression of these proteins in the resistant cell line. When knocking down c-myc in MCF-7/TAM, cells become more sensitive to tamoxifen, cell cycle is blocked as well, indicating that aspirin can regulate c-myc and cyclinD1 proteins to overcome tamoxifen resistance. Our study discovered a novel role of aspirin based on its anti-tumor effect, and put forward some kinds of possible mechanisms of tamoxifen resistance in ER-positive breast cancer cells, providing a new strategy for the treatment of ER-positive breast carcinoma.
Keywords: ER-positive breast cancer; aspirin; c-myc; cyclinD1; tamoxifen resistance.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
A miR-26a/E2F7 feedback loop contributes to tamoxifen resistance in ER-positive breast cancer.Int J Oncol. 2018 Oct;53(4):1601-1612. doi: 10.3892/ijo.2018.4492. Epub 2018 Jul 19. Int J Oncol. 2018. PMID: 30066905
-
Keratinocyte growth factor (KGF) induces tamoxifen (Tam) resistance in human breast cancer MCF-7 cells.Anticancer Res. 2006 May-Jun;26(3A):1773-84. Anticancer Res. 2006. PMID: 16827106
-
Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9.Molecules. 2013 Jan 8;18(1):701-20. doi: 10.3390/molecules18010701. Molecules. 2013. PMID: 23299550 Free PMC article.
-
[Cytotoxicity of tamoxifen and its principal metabolites in human breast cancer cell lines].Bull Cancer. 1996 Oct;83(10):808-15. Bull Cancer. 1996. PMID: 8952630 Review. French.
-
Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors.Curr Opin Pharmacol. 2018 Aug;41:59-65. doi: 10.1016/j.coph.2018.04.009. Epub 2018 Apr 30. Curr Opin Pharmacol. 2018. PMID: 29719270 Free PMC article. Review.
Cited by
-
Salicylate suppresses the oncogenic hyaluronan network in metastatic breast cancer cells.Matrix Biol Plus. 2020 Mar 5;6-7:100031. doi: 10.1016/j.mbplus.2020.100031. eCollection 2020 May. Matrix Biol Plus. 2020. PMID: 33543028 Free PMC article.
-
Taxol‑resistant breast cancer cell‑derived exosome‑delivered miR‑187‑5p regulates the growth of breast cancer cells via ABCD2 and Wnt/β‑catenin signaling.Oncol Lett. 2023 Feb 7;25(3):119. doi: 10.3892/ol.2023.13705. eCollection 2023 Mar. Oncol Lett. 2023. PMID: 36844629 Free PMC article.
-
PKM2-c-Myc-Survivin Cascade Regulates the Cell Proliferation, Migration, and Tamoxifen Resistance in Breast Cancer.Front Pharmacol. 2020 Sep 8;11:550469. doi: 10.3389/fphar.2020.550469. eCollection 2020. Front Pharmacol. 2020. PMID: 33013387 Free PMC article.
-
Telmisartan inhibits NSCLC A549 cell proliferation and migration by regulating the PI3K/AKT signaling pathway.Oncol Lett. 2018 Apr;15(4):5859-5864. doi: 10.3892/ol.2018.8002. Epub 2018 Feb 8. Oncol Lett. 2018. PMID: 29552215 Free PMC article.
-
Tumor-Associated Macrophages Induce Endocrine Therapy Resistance in ER+ Breast Cancer Cells.Cancers (Basel). 2019 Feb 6;11(2):189. doi: 10.3390/cancers11020189. Cancers (Basel). 2019. PMID: 30736340 Free PMC article.
References
-
- Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. - PubMed
-
- Patani N, Martin LA. Understanding response and resistance to oestrogen deprivation in ER-positive breast cancer. Mol Cell Endocrinol. 2014;382:683–694. - PubMed
-
- Wise J. Tamoxifen benefits women who have had invasive breast cancer, community study finds. BMJ. 2016;355:i5442. - PubMed
-
- Chia S, Bryce C, Gelmon K. The 2000 EBCTCG overview: a widening gap. Lancet. 2005;365:1665–1666. - PubMed
-
- Thomas NB, Hutcheson IR, Campbell L, Gee J, Taylor KM, Nicholson RI, Gumbleton M. Growth of hormone-dependent MCF-7 breast cancer cells is promoted by constitutive caveolin-1 whose expression is lost in an EGF-R-mediated manner during development of tamoxifen resistance. Breast Cancer Res Treat. 2010;119:575–591. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

