Type I interferon signaling is required for the APOBEC3/Rfv3-dependent neutralizing antibody response but not innate retrovirus restriction
- PMID: 28415995
- PMCID: PMC5392950
- DOI: 10.1186/s12977-017-0349-2
Type I interferon signaling is required for the APOBEC3/Rfv3-dependent neutralizing antibody response but not innate retrovirus restriction
Abstract
Background: APOBEC3/Rfv3 restricts acute Friend retrovirus (FV) infection and promotes virus-specific neutralizing antibody (NAb) responses. Classical Rfv3 studies utilized FV stocks containing lactate-dehydrogenase elevating virus (LDV), a potent type I interferon inducer. Previously, we showed that APOBEC3 is required for the anti-FV activity of exogenous IFN-alpha treatment. Thus, type I interferon receptor (IFNAR) signaling may be required for the APOBEC3/Rfv3 response.
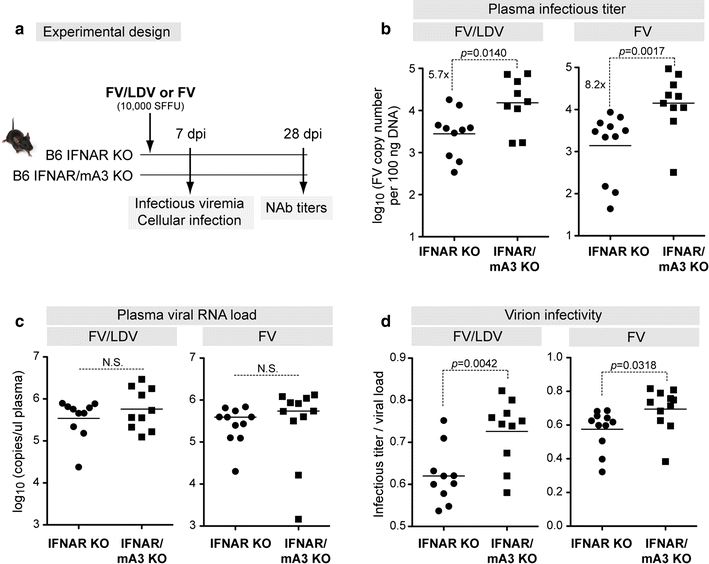
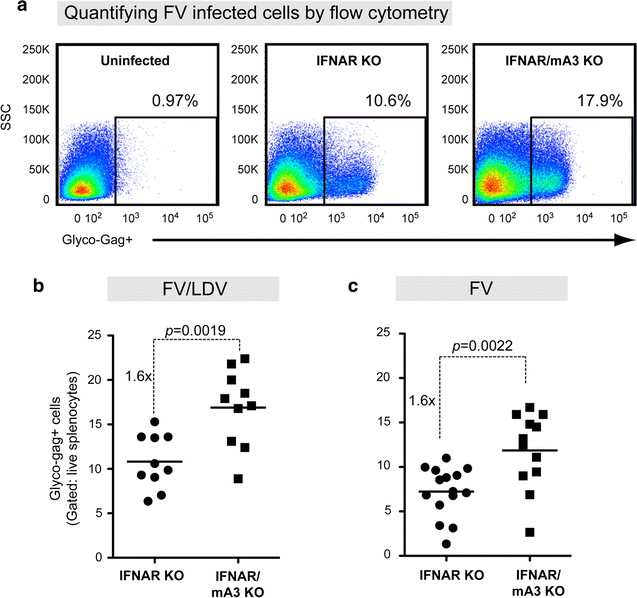
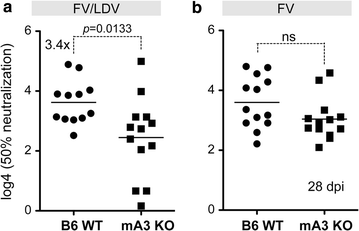
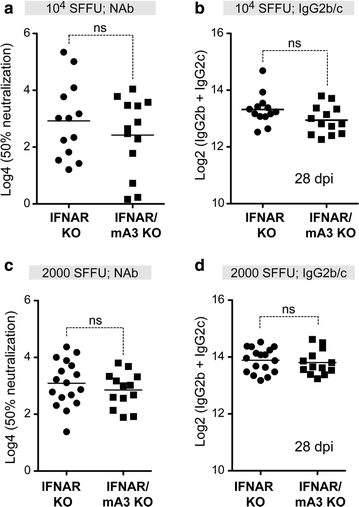
Results: To test if the APOBEC3/Rfv3 response is dependent on type I IFN signaling, we infected IFNAR knockout versus IFNAR/APOBEC3 double-knockout mice with FV/LDV or LDV-free FV, and evaluated acute FV infection and subsequent NAb titers. We show that LDV co-infection and type I IFN signaling are not required for innate APOBEC3-mediated restriction. By contrast, removal of LDV and/or type I IFN signaling abrogated the APOBEC3-dependent NAb response.
Conclusions: APOBEC3 can restrict retroviruses in a type I IFN-independent manner in vivo. By contrast, the ability of APOBEC3 to promote NAb responses is type I IFN-dependent. These findings reveal novel insights on the interplay between type I IFNs and APOBEC3 in vivo that may have implications for augmenting antiretroviral NAb responses.
Keywords: Deaminase; Friend retrovirus; IFNAR; LDV; Neutralizing antibody.
Figures
Similar articles
-
Requirement for Fc effector mechanisms in the APOBEC3/Rfv3-dependent neutralizing antibody response.J Virol. 2015 Apr;89(7):4011-4. doi: 10.1128/JVI.03399-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589647 Free PMC article.
-
Noninfectious retrovirus particles drive the APOBEC3/Rfv3 dependent neutralizing antibody response.PLoS Pathog. 2011 Oct;7(10):e1002284. doi: 10.1371/journal.ppat.1002284. Epub 2011 Oct 6. PLoS Pathog. 2011. PMID: 21998583 Free PMC article.
-
Innate retroviral restriction by Apobec3 promotes antibody affinity maturation in vivo.J Immunol. 2010 Jul 15;185(2):1114-23. doi: 10.4049/jimmunol.1001143. Epub 2010 Jun 21. J Immunol. 2010. PMID: 20566830 Free PMC article.
-
Host genetic factors that control immune responses to retrovirus infections.Vaccine. 2008 Jun 6;26(24):2981-96. doi: 10.1016/j.vaccine.2008.01.004. Epub 2008 Jan 22. Vaccine. 2008. PMID: 18255203 Review.
-
Humoral immunity in the Friend retrovirus infection model.Immunol Res. 2013 Mar;55(1-3):249-60. doi: 10.1007/s12026-012-8370-y. Immunol Res. 2013. PMID: 22961660 Free PMC article. Review.
Cited by
-
APOBEC3 enzymes mediate efficacy of cisplatin and are epistatic with base excision repair and mismatch repair in platinum response.NAR Cancer. 2020 Dec;2(4):zcaa033. doi: 10.1093/narcan/zcaa033. Epub 2020 Nov 6. NAR Cancer. 2020. PMID: 33196045 Free PMC article.
-
Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes.Sci Rep. 2020 Feb 24;10(1):3229. doi: 10.1038/s41598-020-60213-0. Sci Rep. 2020. PMID: 32094377 Free PMC article.
-
Mouse APOBEC3 Restriction of Retroviruses.Viruses. 2020 Oct 27;12(11):1217. doi: 10.3390/v12111217. Viruses. 2020. PMID: 33121095 Free PMC article. Review.
-
Friend retrovirus studies reveal complex interactions between intrinsic, innate and adaptive immunity.FEMS Microbiol Rev. 2019 Sep 1;43(5):435-456. doi: 10.1093/femsre/fuz012. FEMS Microbiol Rev. 2019. PMID: 31087035 Free PMC article. Review.
-
Transcriptional signatures associated with rubella virus-specific humoral immunity after a third dose of MMR vaccine in women of childbearing age.Eur J Immunol. 2021 Jul;51(7):1824-1838. doi: 10.1002/eji.202049054. Epub 2021 Apr 19. Eur J Immunol. 2021. PMID: 33818775 Free PMC article.
References
-
- Meager A. The interferons: characterization and application. Germany: Wiley-VCH; 2006.
-
- Santiago ML, Greene WC. The role of the Apobec3 family of cytidine deaminases in innate immunity, G-to-A hypermutation and evolution of retroviruses. In: Domingo E, Parrish CR, Holland JJ, editors. Origin and evolution of viruses. London: Academic Press; 2008. pp. 183–206.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

