Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis
- PMID: 28416077
- PMCID: PMC5474951
- DOI: 10.1016/j.neuron.2017.03.043
Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis
Abstract
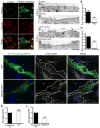
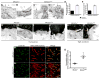
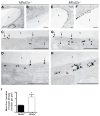
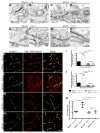
The blood-brain barrier (BBB) provides a constant homeostatic brain environment that is essential for proper neural function. An unusually low rate of vesicular transport (transcytosis) has been identified as one of the two unique properties of CNS endothelial cells, relative to peripheral endothelial cells, that maintain the restrictive quality of the BBB. However, it is not known how this low rate of transcytosis is achieved. Here we provide a mechanism whereby the regulation of CNS endothelial cell lipid composition specifically inhibits the caveolae-mediated transcytotic route readily used in the periphery. An unbiased lipidomic analysis reveals significant differences in endothelial cell lipid signatures from the CNS and periphery, which underlie a suppression of caveolae vesicle formation and trafficking in brain endothelial cells. Furthermore, lipids transported by Mfsd2a establish a unique lipid environment that inhibits caveolae vesicle formation in CNS endothelial cells to suppress transcytosis and ensure BBB integrity.
Keywords: CNS endothelial cells; Cav-1; DHA; Mfsd2a; blood vessels; blood-brain barrier; caveolae; lipid transport; lipidomic mass spectrometry; transcytosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circulation research. 2007;100:158–173. - PubMed
-
- Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. - PubMed
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
-
- Bastiani M, Parton RG. Caveolae at a glance. Journal of cell science. 2010;123:3831–3836. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

